GRAY SALT by DONG IL PHARMS CO.,LTD / DONG IL PHARMS CO., LTD.
GRAY SALT by
Drug Labeling and Warnings
GRAY SALT by is a Otc medication manufactured, distributed, or labeled by DONG IL PHARMS CO.,LTD, DONG IL PHARMS CO., LTD.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
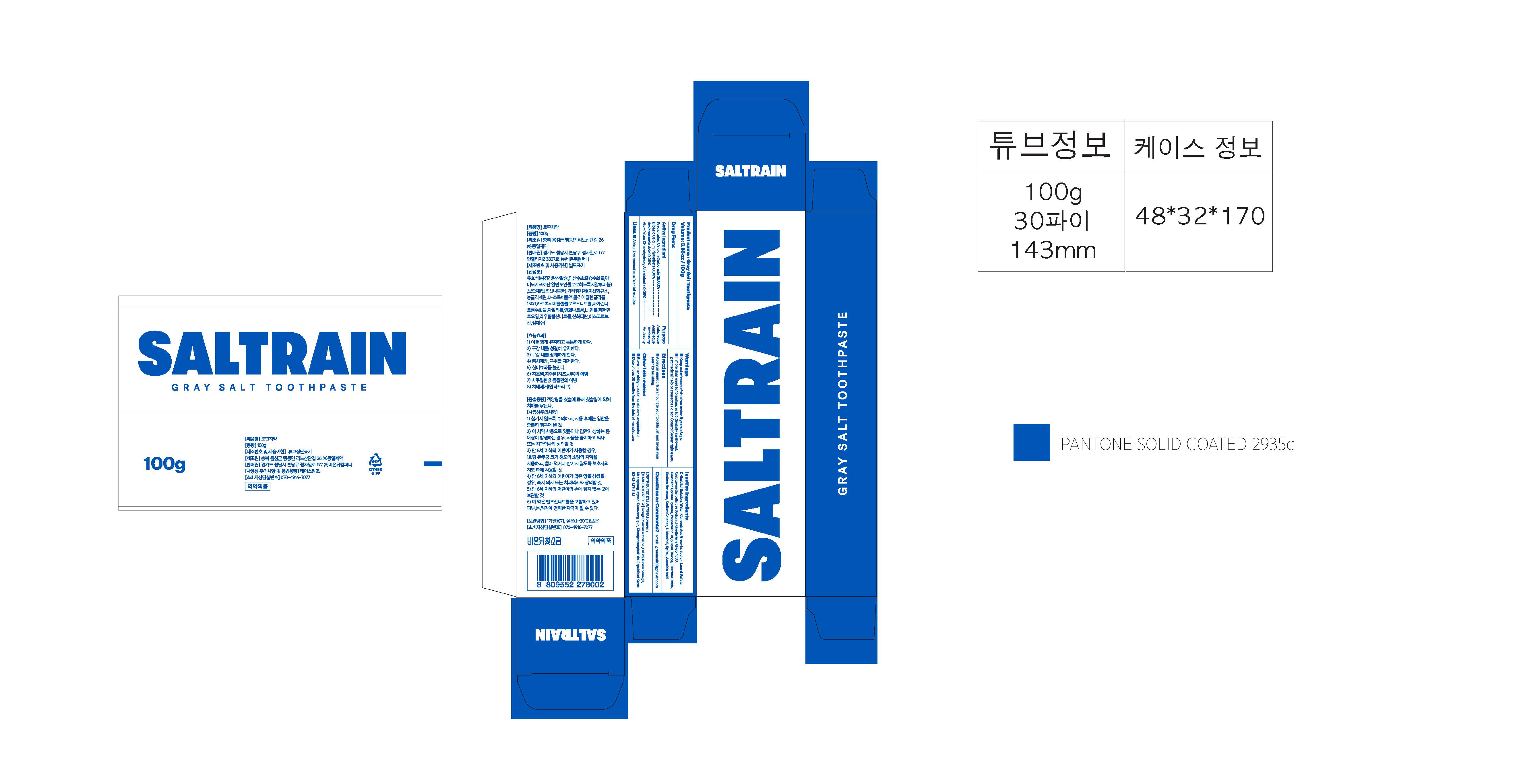
GRAY SALT- precipitated calcium carbonate, dibasic calcium phosphate, aminocaproic acid, aluminium chlorohydroxy allantoinate paste, dentifrice
DONG IL PHARMS CO.,LTD
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Precipitated Calcium Carbonate, Dibasic Calcium Phosphate, Aminocaproic Acid, Aluminium Chlorohydroxy Allantoinate
Storage method
1. Keep it at room temperature in a classified container.
2. Cover and store at room temperature.
3. Store in a not moisture and cool place.
4. Air may come out during use of this product, but there is no problem with its weight.
Usage Precautions
1. Be careful not to swallow. Rinse mouth thoroughly after use
2. If the use of toothpaste causes abnormalities such as gums or mouth injury, discontinue use and consult a doctor or dentist.
3. For children under 6 years of age, use a small amount of toothpaste as small as pea per use, and use under the guidance of a guardian to avoid sucking or swallowing.
4. If a child under 6 years old swallows large amount, consult with a doctor or dentist immediately.
5. Keep out of the reach of children under 6 years of age.
| GRAY SALT
precipitated calcium carbonate, dibasic calcium phosphate, aminocaproic acid, aluminium chlorohydroxy allantoinate paste, dentifrice |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - DONG IL PHARMS CO.,LTD (557810721) |
| Registrant - DONG IL PHARMS CO.,LTD (557810721) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| DONG IL PHARMS CO., LTD. | 557810721 | manufacture(73242-0104) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.