Non-medicated VALCHLOR® Demonstration Gel
Non-medicated VALCHLOR Demonstration by
Drug Labeling and Warnings
Non-medicated VALCHLOR Demonstration by is a Prescription medication manufactured, distributed, or labeled by Actelion Pharmaceuticals US, Inc., Actelion Pharmaceuticals, Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
NON-MEDICATED VALCHLOR DEMONSTRATION- non-medicated valchlor demonstration gel
Actelion Pharmaceuticals US, Inc.
----------
Non-medicated VALCHLOR® Demonstration Gel
Instructions For Use1
Non-medicated VALCHLOR® Demonstration Gel DOES NOT contain mechlorethamine (the medicine in VALCHLOR).
The purpose of VALCHLOR Demonstration Gel is to show healthcare providers and patients how to properly apply VALCHLOR. This will also allow the user to experience the consistency of VALCHLOR and how it may feel on the skin.
This information does not take the place of talking to your healthcare provider about your condition or your treatment.
How should VALCHLOR Demonstration Gel be used?
- Use VALCHLOR Demonstration Gel on clean and dry, healthy skin
- If you take a bath or shower, be sure to use the gel after your skin is dry (at least 30 minutes after bathing or showering)
How to use VALCHLOR Demonstration Gel:
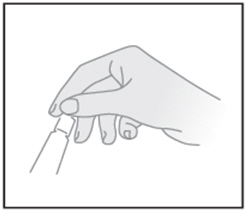 | STEP 1: Open the 5g tube, use cap to puncture seal |
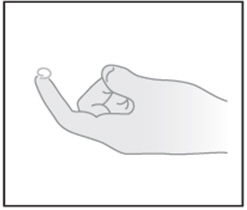 | STEP 2: Squeeze a small pea-sized amount on your fingertip |
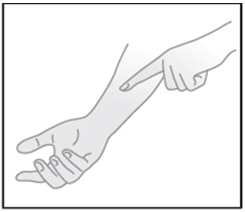 | STEP 3: Gently spread a thin layer of the gel to dry healthy skin |
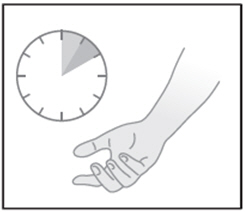 | STEP 4: Allow the gel to dry before covering with clothing (5 to 10 minutes after applying) |
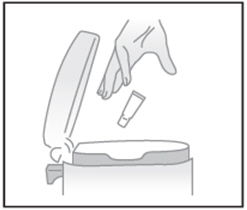 | STEP 5: Throw the tube away in the household trash |
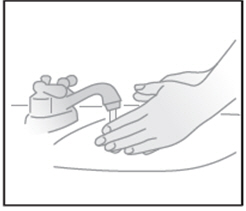 | STEP 6: Wash your hands with soap and water |
Some Important Do's and Don'ts about VALCHLOR Demonstration Gel:
- When applying VALCHLOR Demonstration Gel, avoid the eyes, mouth, and nose.
- VALCHLOR Demonstration Gel should only be used on healthy skin since it is non-medicated.
- The 5g tube of VALCHLOR Demonstration Gel is meant for single use only.
- VALCHLOR Demonstration Gel contains alcohol. Since alcohol-based gels are flammable, avoid fire, flame, and smoking until the gel has dried.
- VALCHLOR Demonstration Gel can be stored at room temperature.
- Keep out of reach of children.
This demonstration gel contains the following nonactive ingredients: diethylene glycol monoethyl ether, propylene glycol, isopropyl alcohol, glycerin, lactic acid, hydroxypropylcellulose, sodium chloride, menthol, edetate disodium, and butylated hydroxytoluene.
Please see VALCHLOR full Prescribing Information including Medication Guide for information for active drug product.
To report SUSPECTED ADVERSE REACTIONS to the VALCHLOR Demonstration Gel, contact Actelion Pharmaceuticals US, Inc., at 1-855-4-VALCHLOR (1-855-482-5245) or FDA at 1-800-FDA-1088 or visit www.fda.gov/medwatch.
The NDC code for the Non-medicated VALCHLOR Demonstration Gel is 66215-001-05.
ACT20151217
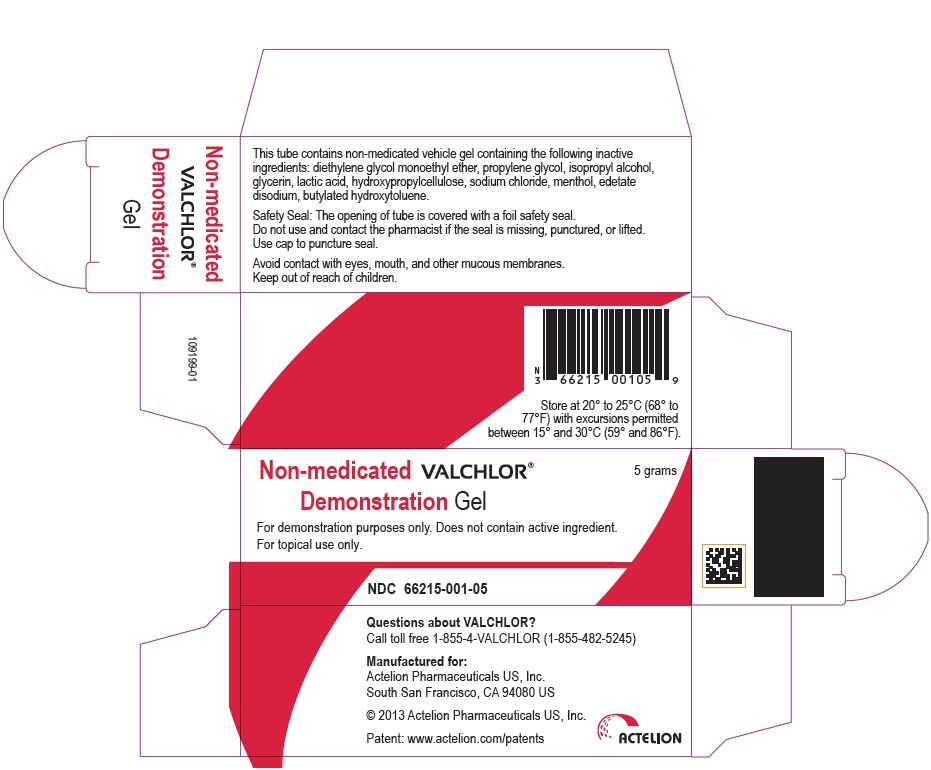
PRINCIPAL DISPLAY PANEL - 5 g Tube Carton
Non-medicated VALCHLOR®
Demonstration Gel
5 grams
For demonstration purposes only. Does not contain active ingredient.
For topical use only.
NDC: 66215-001-05
| NON-MEDICATED VALCHLOR DEMONSTRATION
non-medicated valchlor demonstration gel |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Actelion Pharmaceuticals US, Inc. (002641228) |
| Registrant - Actelion Pharmaceuticals, Ltd. (480007868) |