Alcohol Wet Wipes, 70 Alcohol
Alcohol Wet Wipes, 70 Alcohol by
Drug Labeling and Warnings
Alcohol Wet Wipes, 70 Alcohol by is a Otc medication manufactured, distributed, or labeled by Sweda Company LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ALCOHOL WET WIPES, 70 ALCOHOL- alcohol cloth
Sweda Company LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
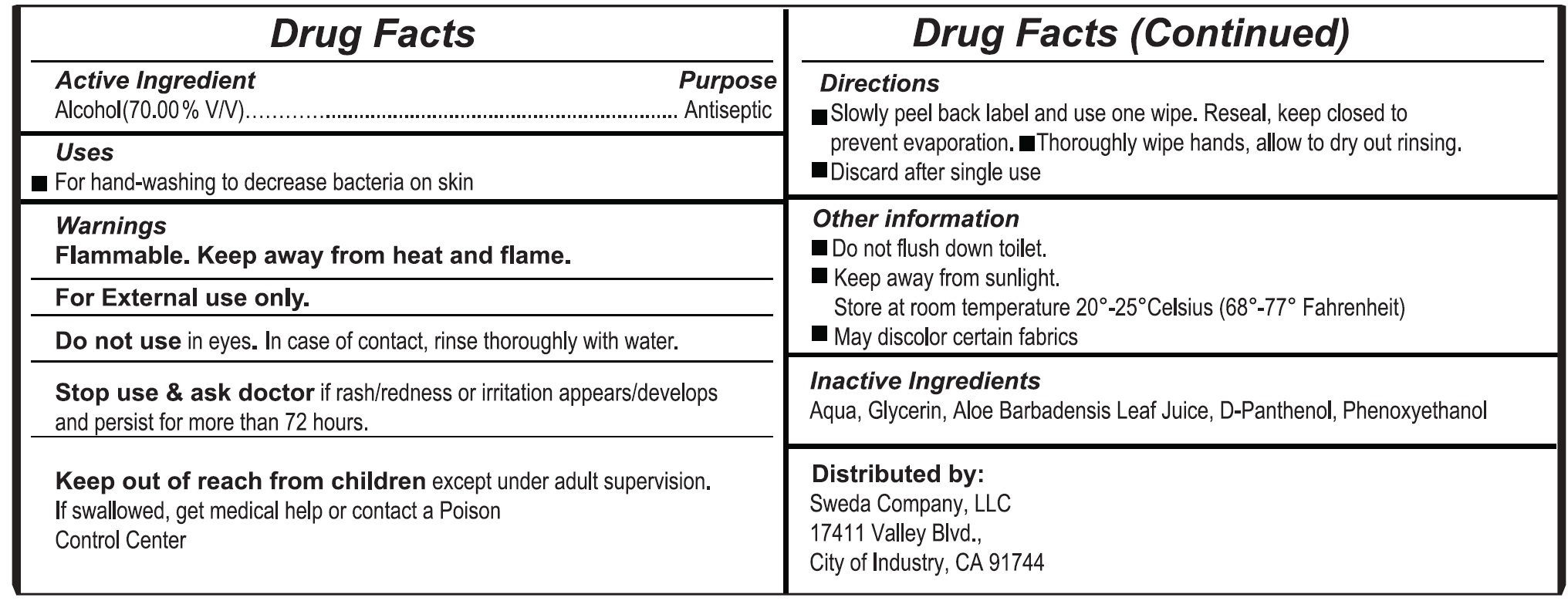
Alcohol Wet Wipes, 70 Alcohol
Warnings
Flammable. Keep away from heat and flame.
For External use only
Directions
- Slowly peel back label and use one wipe. Reseal, keep closed to prevent evaporation.
- Thoroughly wipe hands, allow to dry out rinsing. Discard after single use
| ALCOHOL WET WIPES, 70 ALCOHOL
alcohol cloth |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Sweda Company LLC (081729899) |
Revised: 2/2021
Document Id: bb61c361-fd3a-1748-e053-2995a90a7153
Set id: ad53cd9a-64fb-9af3-e053-2995a90ae15d
Version: 2
Effective Time: 20210215
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.