Antibacterial Wipes by CARSON’S NUT-BOLT AND TOOL COMPANY INCORPORATED
Antibacterial Wipes by
Drug Labeling and Warnings
Antibacterial Wipes by is a Otc medication manufactured, distributed, or labeled by CARSON’S NUT-BOLT AND TOOL COMPANY INCORPORATED. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
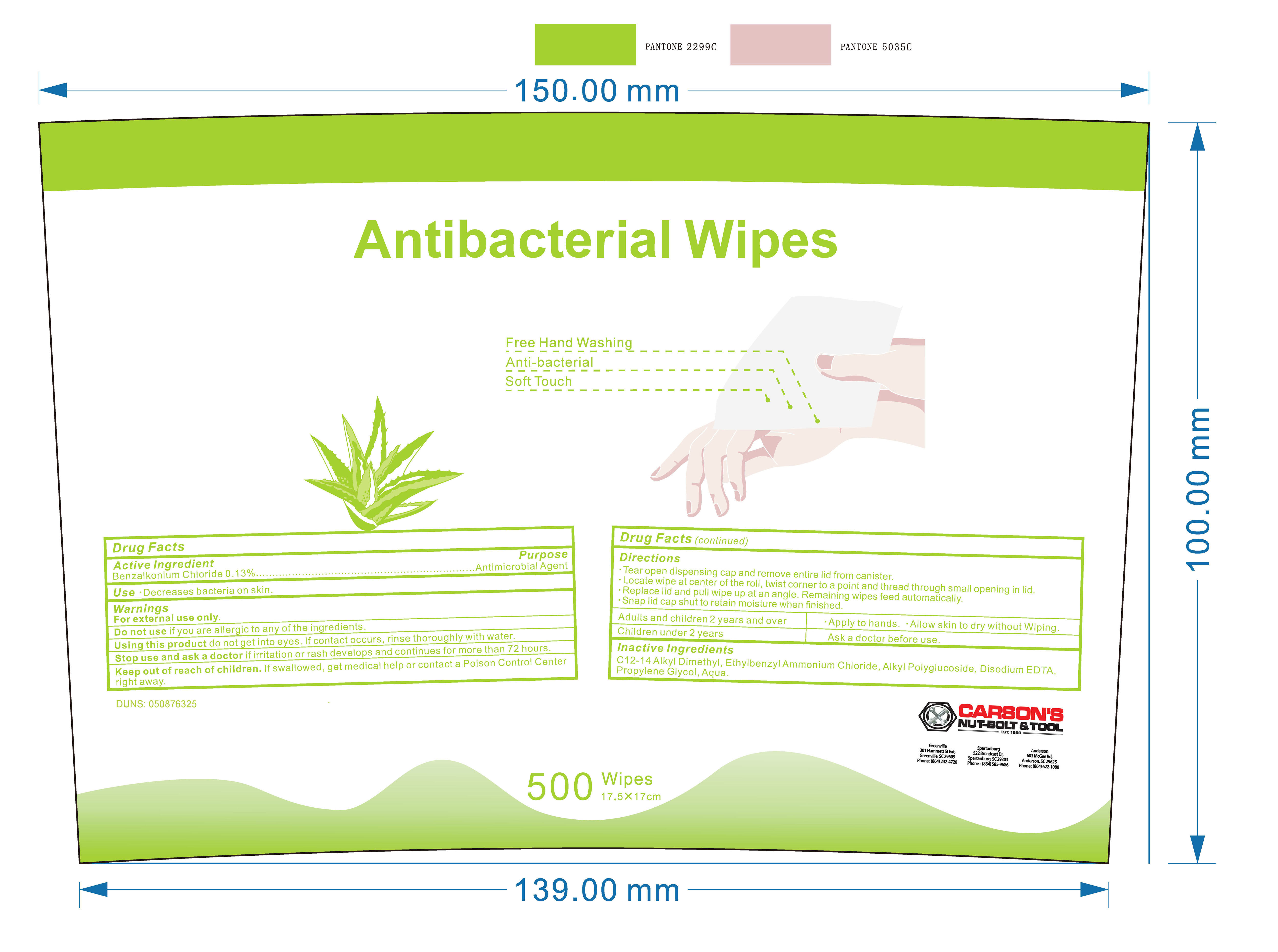
ANTIBACTERIAL WIPES- benzalkonium chloride cloth
CARSON’S NUT-BOLT AND TOOL COMPANY INCORPORATED
----------
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
-Tear open dispensing cap and remove entire lid from canister.
-Locate wipe at center of the roll,twist corner to a point and thread through small opening in lid.
-Replace lid and pull wipe up at an angle.Remaining wipes feed automatically.
-Snap lid cap shut to retain moisture when finished.
| ANTIBACTERIAL WIPES
benzalkonium chloride cloth |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - CARSON’S NUT-BOLT AND TOOL COMPANY INCORPORATED (050876325) |
Revised: 12/2025
Document Id: 47285338-04e5-0072-e063-6294a90aa099
Set id: ad578d1a-ea91-8345-e053-2a95a90a0d8c
Version: 3
Effective Time: 20251230
CARSON’
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.