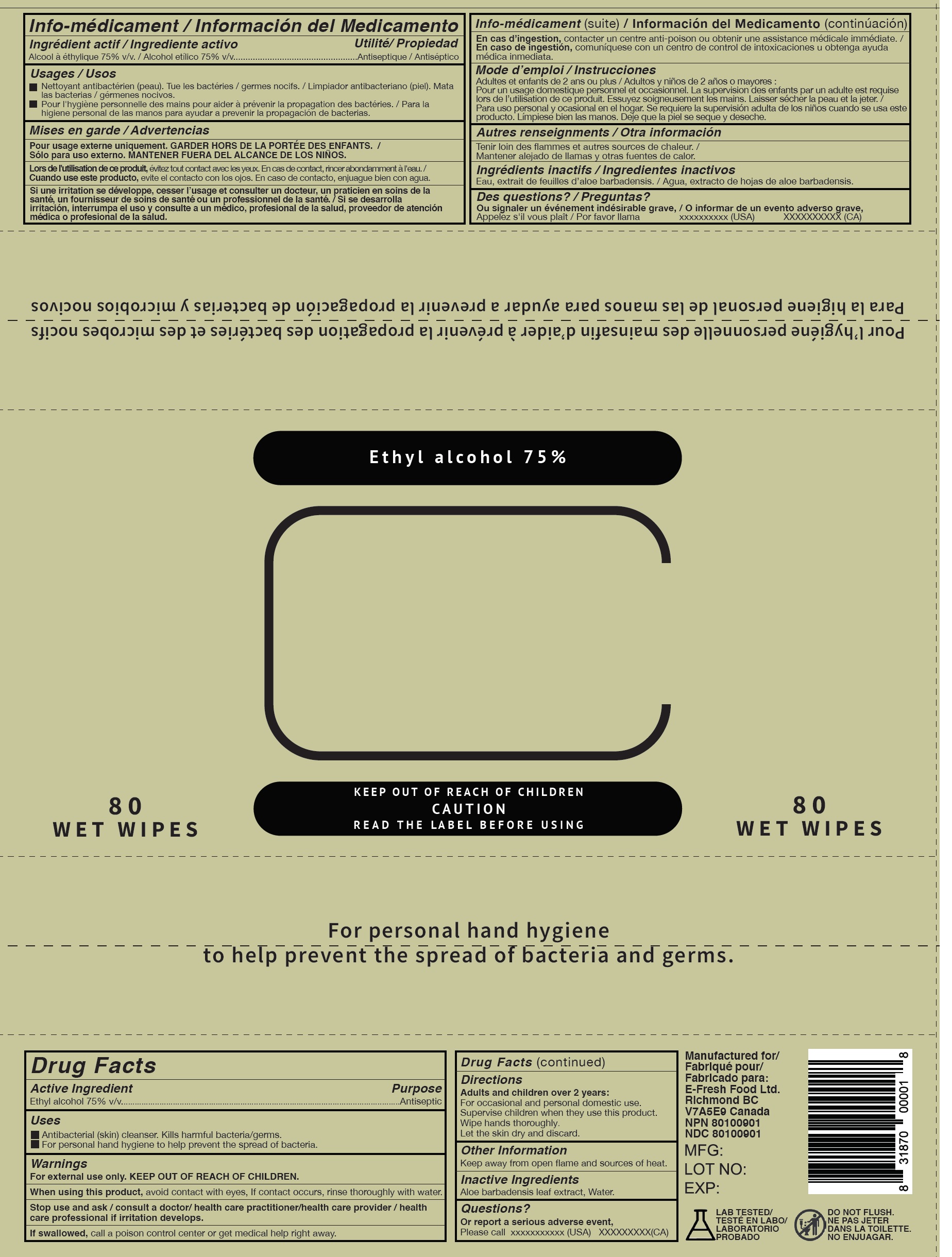
75 Alcohol Hand Sanitizing Wipes
75 Alcohol Hand Sanitizing Wipes by
Drug Labeling and Warnings
75 Alcohol Hand Sanitizing Wipes by is a Otc medication manufactured, distributed, or labeled by E-Fresh Food Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
75 ALCOHOL HAND SANITIZING WIPES- alcohol cloth
E-Fresh Food Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
75 Alcohol Hand Sanitizing Wipes
Uses
- Antibacterial (skin) cleanser. Kills harmful bacteria/germs.
- For personal hand hygiene to help prevent the spread of bacteria.
Warnings
For external use only.
| 75 ALCOHOL HAND SANITIZING WIPES
alcohol cloth |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - E-Fresh Food Ltd. (203413216) |
Revised: 10/2021
Document Id: cea488f7-8040-8668-e053-2a95a90ad3fc
Set id: ad66bad7-d3a8-0c8d-e053-2a95a90ab2f4
Version: 3
Effective Time: 20211018
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.