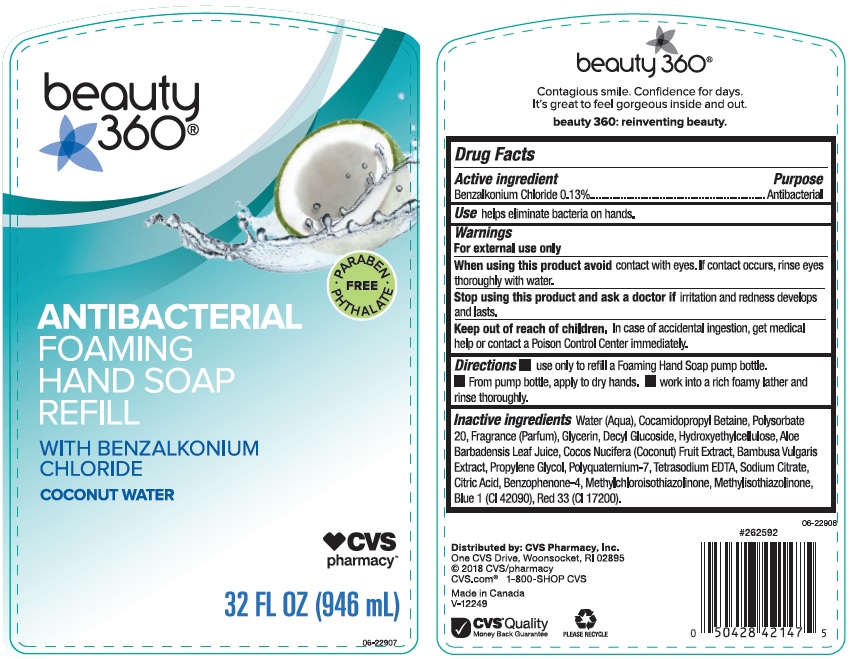
CVS PHARMACY COCONUT WATER- benzalkonium chloride liquid
CVS Pharmacy by
Drug Labeling and Warnings
CVS Pharmacy by is a Otc medication manufactured, distributed, or labeled by CVS Pharmacy, Apollo Health and Beauty Care Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient
- Use
- Warnings
- Directions
-
Inactive ingredients
Water (Aqua), Cocamidopropyl Betaine, Polysorbate 20, Fragrance (Parfum), Glycerin, Decyl Glucoside, Hydroxyethylcellulose, Aloe Barbadensis Leaf Juice, Cocos Nucifera (Coconut) Fruit Extract, Bambusa Vulgaris Extract, Propylene Glycol, Polyquaternium-7, Tetrasodium EDTA, Sodium Citrate, Citric Acid, Benzophenone-4, Methylchloroisothiazolinone, Methylisothiazolinone, Blue 1 (CI 42090), Red 33 (CI 17200).
- Label Copy
-
INGREDIENTS AND APPEARANCE
CVS PHARMACY COCONUT WATER
benzalkonium chloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 59779-842 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 1.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) POLYSORBATE 20 (UNII: 7T1F30V5YH) GLYCERIN (UNII: PDC6A3C0OX) DECYL GLUCOSIDE (UNII: Z17H97EA6Y) HYPROMELLOSES (UNII: 3NXW29V3WO) ALOE VERA LEAF (UNII: ZY81Z83H0X) COCONUT (UNII: 3RT3536DHY) BAMBUSA VULGARIS TOP (UNII: FIW80T6P6V) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POLYQUATERNIUM-7 (70/30 ACRYLAMIDE/DADMAC; 1600000 MW) (UNII: 0L414VCS5Y) EDETATE SODIUM (UNII: MP1J8420LU) SODIUM CITRATE (UNII: 1Q73Q2JULR) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SULISOBENZONE (UNII: 1W6L629B4K) METHYLCHLOROISOTHIAZOLINONE (UNII: DEL7T5QRPN) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C RED NO. 33 (UNII: 9DBA0SBB0L) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59779-842-33 946 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/04/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 07/04/2018 Labeler - CVS Pharmacy (062312574) Registrant - Apollo Health and Beauty Care Inc. (201901209) Establishment Name Address ID/FEI Business Operations Apollo Health and Beauty Care Inc. 201901209 manufacture(59779-842)
Trademark Results [CVS Pharmacy]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CVS PHARMACY 97287242 not registered Live/Pending |
CVS Pharmacy, Inc. 2022-02-28 |
 CVS PHARMACY 87622772 5453318 Live/Registered |
CVS Pharmacy, Inc. 2017-09-26 |
 CVS PHARMACY 86893256 5031926 Live/Registered |
CVS Pharmacy, Inc. 2016-02-01 |
 CVS PHARMACY 86892916 5027198 Live/Registered |
CVS Pharmacy, Inc. 2016-02-01 |
 CVS PHARMACY 86882356 5157603 Live/Registered |
CVS Pharmacy, Inc. 2016-01-21 |
 CVS PHARMACY 86879078 5157590 Live/Registered |
CVS Pharmacy, Inc. 2016-01-19 |
 CVS PHARMACY 86123747 5022909 Live/Registered |
CVS Pharmacy, Inc. 2013-11-20 |
 CVS PHARMACY 86091649 4608526 Live/Registered |
CVS Pharmacy, Inc. 2013-10-15 |
 CVS PHARMACY 86064266 4591680 Live/Registered |
CVS Pharmacy, Inc. 2013-09-13 |
 CVS PHARMACY 86025887 4673785 Live/Registered |
CVS Pharmacy, Inc. 2013-08-01 |
 CVS PHARMACY 76372220 2774665 Live/Registered |
CVS PHARMACY, INC. 2002-02-19 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.