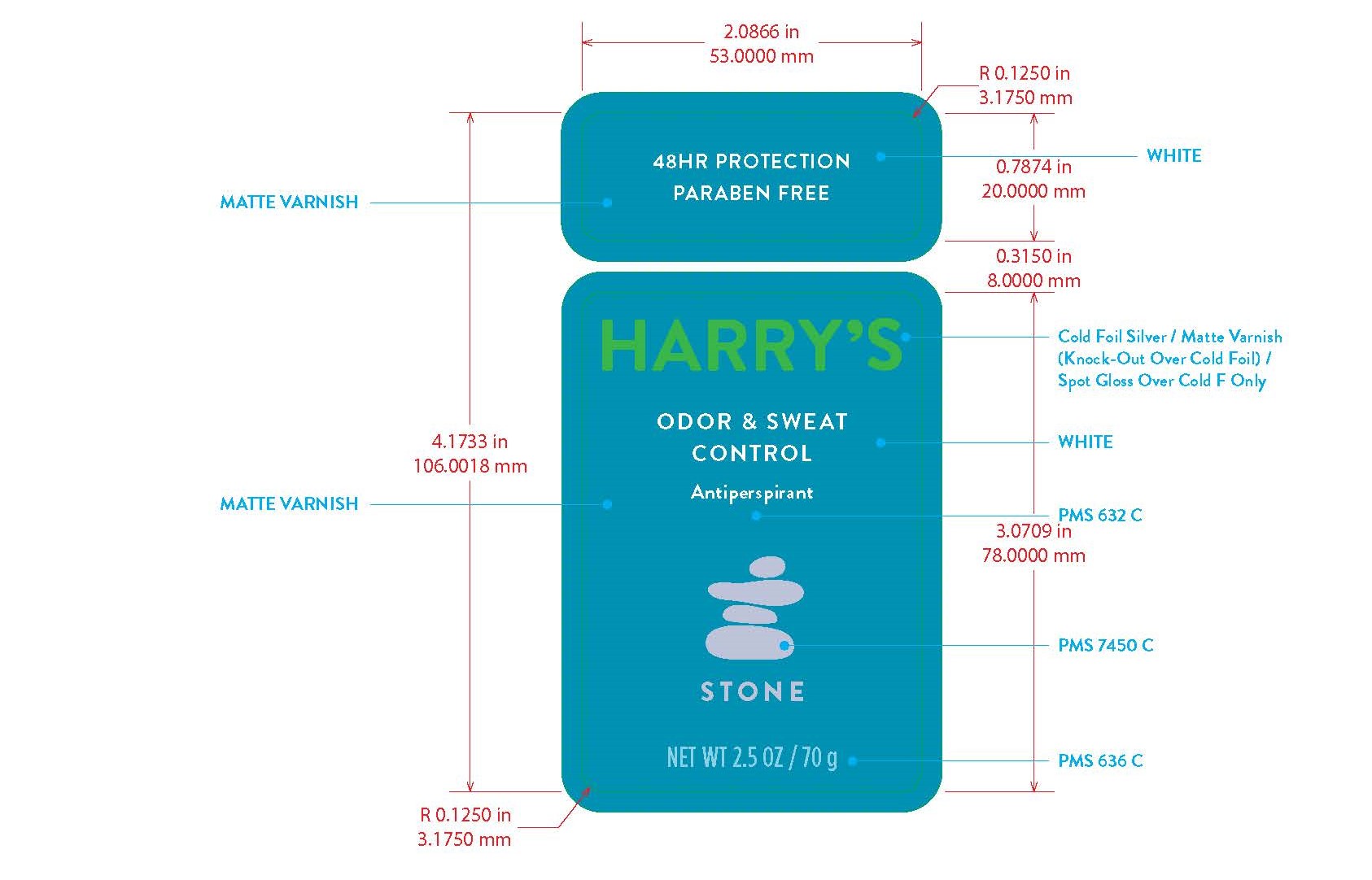
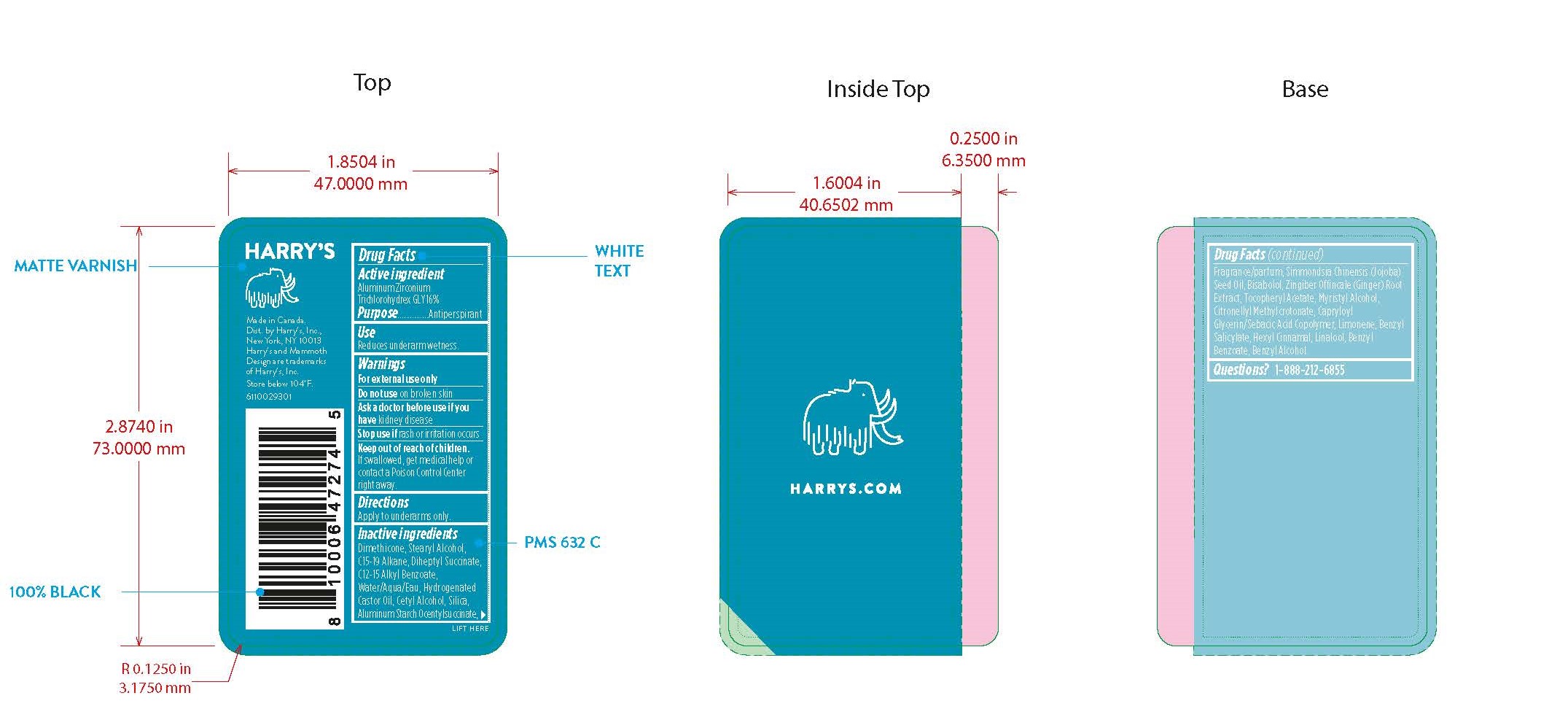
Harrys Antiperspirant Stone by Harry's Inc. AP - Stone
Harrys Antiperspirant Stone by
Drug Labeling and Warnings
Harrys Antiperspirant Stone by is a Otc medication manufactured, distributed, or labeled by Harry's Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
HARRYS ANTIPERSPIRANT STONE- aluminum zirconium trichlorohydrex gly stick
Harry's Inc.
----------
AP - Stone
Inactive Ingredients
DIMETHICONE,STEARYL ALCOHOL, C15-19 ALKANE, DIHEPTYL SUCCINATE, C12-15 ALKYL BENZOATE, WATER/AQUA/EAU, HYDROGENATED CASTOR OIL, CETYL ALCOHOL, SILICA, ALUMINUM STARCH OCENTYLSUCCINATE, FRAGRANCE/PARFUM, SIMMONDSIA CHINENSIS (JOJOBA) SEED OIL, BISABOLOL, ZINGIBER OFFINCALE (GINGER) ROOT EXTRACT, TOCOPHERYL ACETATE, MYRISTYL ALCOHOL, CITRONELLYL METHYLCROTONATE, CAPRYLOYL GLYCERIN/SEBACIC ACID COPOLYMER, LIMONENE, BENZYL SALICYLATE, HEXYL CINNAMAL, LINALOOL, BENZYL BENZOATE, BENZYL ALCOHOL.
| HARRYS ANTIPERSPIRANT STONE
aluminum zirconium trichlorohydrex gly stick |
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Harry's Inc. (079239206) |
Revised: 12/2025
<
Document Id: 463e8e28-36c1-987a-e063-6394a90a47e7
Set id: adc9efbb-6605-596e-e053-2a95a90a0835
Version: 5
Effective Time: 20251218
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.