Harrys Antiperspirant Shiso by Harry's Inc. AP - Shiso
Harrys Antiperspirant Shiso by
Drug Labeling and Warnings
Harrys Antiperspirant Shiso by is a Otc medication manufactured, distributed, or labeled by Harry's Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
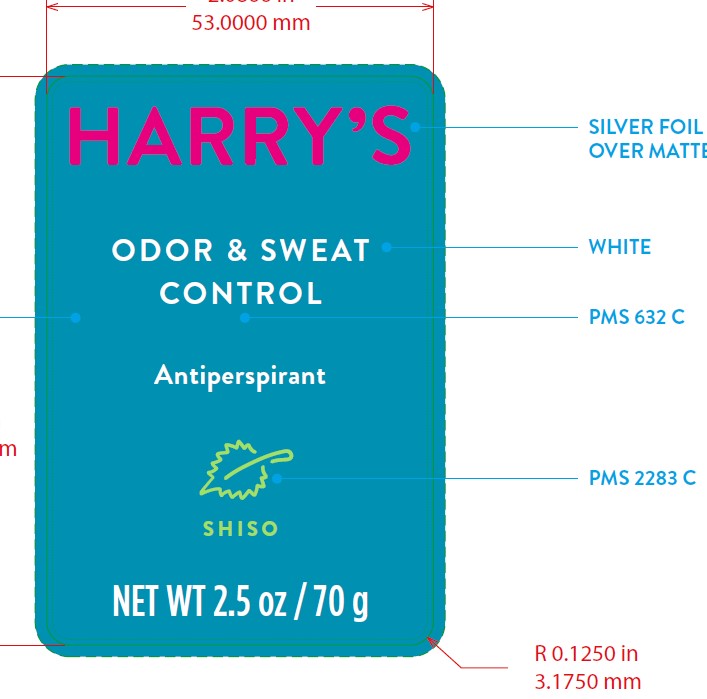
HARRYS ANTIPERSPIRANT SHISO- aluminum zirconium trichlorohydrex gly stick
Harry's Inc.
----------
AP - Shiso
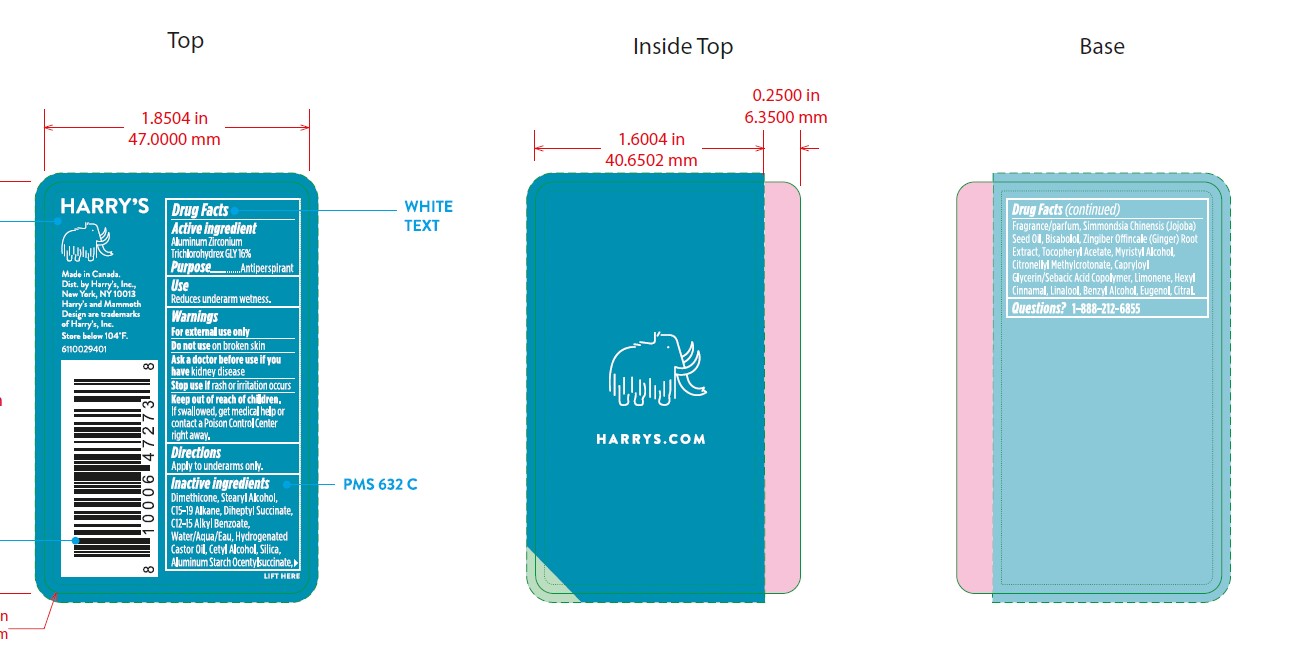
Inactive Ingredients
DIMETHICONE, STEARYL ALCOHOL, C15-19 ALKANE, DIHEPTYL SUCCINATE, C12-15 ALKYL BENZOATE, WATER/AQUA/EAU, HYDROGENATED CASTOR OIL, CETYL ALCOHOL, SILICA, ALUMINUM STARCH OCENTYLSUCCINATE, FRAGRANCE/PARFUM, SIMMONDSIA CHINENSIS (JOJOBA) SEED OIL, BISABOLOL, ZINGIBER OFFINCALE (GINGER) ROOT EXTRACT, TOCOPHERYL ACETATE, MYRISTYL ALCOHOL, CITRONELLYL METHYLCROTONATE, CAPRYLOYL GLYCERIN/SEBACIC ACID COPOLYMER, LIMONENE, HEXYL CINNAMAL, LINALOOL, BENZYL ALCOHOL, EUGENOL, CITRAL.
| HARRYS ANTIPERSPIRANT SHISO
aluminum zirconium trichlorohydrex gly stick |
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Harry's Inc. (079239206) |
Revised: 12/2025
<
Document Id: 463e81fd-f9ca-9062-e063-6394a90ade99
Set id: adcc77d1-41b2-6c3c-e053-2995a90a1466
Version: 5
Effective Time: 20251218
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.