ALIQOPA- copanlisib injection, powder, lyophilized, for solution
ALIQOPA by
Drug Labeling and Warnings
ALIQOPA by is a Prescription medication manufactured, distributed, or labeled by Bayer HealthCare Pharmaceuticals Inc., Sharp Corporation, Bayer AG. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ALIQOPA safely and effectively. See full prescribing information for ALIQOPA.
ALIQOPA™ (copanlisib) for injection, for intravenous use
Initial U.S. Approval: 2017RECENT MAJOR CHANGES
Dosage and Administration (2.2) 10/2019
INDICATIONS AND USAGE
ALIQOPA is a kinase inhibitor indicated for the treatment of adult patients with relapsed follicular lymphoma (FL) who have received at least two prior systemic therapies (1).
Accelerated approval was granted for this indication based on overall response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
DOSAGE AND ADMINISTRATION
- Recommended dosage: 60 mg administered as a 1-hour intravenous infusion on Days 1, 8, and 15 of a 28-day treatment cycle on an intermittent schedule (three weeks on and one week off). Modify dosage for toxicity (2.1, 2.4).
- Reduce the ALIQOPA dose to 45 mg in patients with moderate hepatic impairment (Child-Pugh B) (2.2, 8.6).
- See full prescribing information for important preparation and administration information (2.5, 2.6, 2.7).
DOSAGE FORMS AND STRENGTHS
For injection: 60 mg as a lyophilized solid in single-dose vial for reconstitution (3).
CONTRAINDICATIONS
None (4).
WARNINGS AND PRECAUTIONS
- Infections: Monitor patients for signs and symptoms of infection. Withhold treatment for Grade 3 and higher infections until resolution (5.1).
- Hyperglycemia: Start each infusion once optimal blood glucose control is achieved. Withhold treatment, reduce dose, or discontinue treatment depending on the severity and persistence of hyperglycemia (5.2).
- Hypertension: Withhold treatment in patients until both the systolic blood pressure (BP) is less than 150 mmHg and the diastolic BP is less than 90 mmHg. Consider reducing dose if anti-hypertensive treatment is required. Discontinue in patients with BP that is uncontrolled or with life-threatening consequences (5.3).
- Non-infectious pneumonitis (NIP): Treat NIP and reduce dose. Discontinue treatment if Grade 2 NIP recurs or in patients experiencing Grade 3 or higher NIP (5.4).
- Neutropenia: Monitor blood counts at least weekly while under treatment. Withhold treatment until ANC ≥0.5 x 103 cells/mm3 (5.5).
- Severe Cutaneous Reactions: Withhold treatment, reduce dose, or discontinue treatment depending on the severity and persistence of severe cutaneous reactions (5.6).
- Embryo-Fetal Toxicity: ALIQOPA can cause fetal harm. Advise patients of potential risk to a fetus and to use effective contraception (5.7, 8.1, 8.3).
ADVERSE REACTIONS
The most common adverse reactions (≥20%) are hyperglycemia, diarrhea, decreased general strength and energy, hypertension, leukopenia, neutropenia, nausea, lower respiratory tract infections, thrombocytopenia (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Bayer Healthcare Pharmaceuticals Inc. at 1-888-842-2937 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 2/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Dose Modification for Moderate Hepatic Impairment
2.3 Dose Modification for Use with Strong CYP3A Inhibitors
2.4 Dose Modification for Toxicities
2.5 Preparation and Administration
2.6 Reconstitution Instructions
2.7 Dilution Instructions for Intravenous Use
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Infections
5.2 Hyperglycemia
5.3 Hypertension
5.4 Non-Infectious Pneumonitis
5.5 Neutropenia
5.6 Severe Cutaneous Reactions
5.7 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on Copanlisib
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Relapsed Follicular Lymphoma
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
ALIQOPA is indicated for the treatment of adult patients with relapsed follicular lymphoma (FL) who have received at least two prior systemic therapies.
- Accelerated approval was granted for this indication based on overall response rate [see Clinical Studies (14.1)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dose of ALIQOPA is 60 mg administered as a 1-hour intravenous infusion on Days 1, 8, and 15 of a 28-day treatment cycle on an intermittent schedule (three weeks on and one week off). Continue treatment until disease progression or unacceptable toxicity [see Warnings and Precautions (5)].
2.2 Dose Modification for Moderate Hepatic Impairment
Reduce ALIQOPA dose to 45 mg in patients with moderate hepatic impairment (Child-Pugh B) [see Hepatic Impairment (8.6)].
2.3 Dose Modification for Use with Strong CYP3A Inhibitors
Reduce ALIQOPA dose to 45 mg if a strong CYP3A inhibitor must be used. Concomitant use of ALIQOPA with strong CYP3A inhibitors increases copanlisib exposure (AUC) and may increase the risk for toxicity [see Drug Interactions (7.1)].
2.4 Dose Modification for Toxicities
Manage toxicities per Table 1 with dose reduction, treatment delay, or discontinuation of ALIQOPA. Discontinue ALIQOPA if life-threatening ALIQOPA-related toxicity occurs.
Table 1: Dose Modification and Toxicity Managementa Toxicities Adverse Reaction Gradeb Recommended Management Infections
Grade 3 or higher
Withhold ALIQOPA until resolution.
Suspected pneumocystis jiroveci pneumonia (PJP) infection of any grade
Withhold ALIQOPA. If confirmed, treat infection until resolution, then resume ALIQOPA at previous dose with concomitant PJP prophylaxis.
Hyperglycemia
Pre-dose fasting blood glucose
160 mg/dL or more or random/non-fasting blood glucose of 200 mg/dL or moreWithhold ALIQOPA until fasting glucose is 160 mg/dL or less, or a random/non-fasting blood glucose of 200 mg/dL or less.
Pre-dose or post-dose blood glucose 500 mg/dL or more
On first occurrence, withhold ALIQOPA until fasting blood glucose is 160 mg/dL or less, or a random/non-fasting blood glucose of 200 mg/dL or less. Then reduce ALIQOPA from 60 mg to 45 mg and maintain.
On subsequent occurrences, withhold ALIQOPA until fasting blood glucose is 160 mg/dL or less, or a random/non-fasting blood glucose of 200 mg/dL or less. Then reduce ALIQOPA from 45 mg to 30 mg and maintain. If persistent at 30 mg, discontinue ALIQOPA.
Hypertension
Pre-dose blood pressure (BP) 150/90 or greaterc
Withhold ALIQOPA until BP is less than 150/90 based on two consecutive BP measurements at least 15 minutes apart.
Post-dose BP 150/90 or greaterc (non-life-threatening):
If anti-hypertensive treatment is not required, continue ALIQOPA at previous dose. If anti-hypertensive treatment is required, consider reduction of ALIQOPA from 60 mg to 45 mg or from 45 mg to 30 mg. Discontinue ALIQOPA if BP remains uncontrolled (BP greater than 150/90) despite anti-hypertensive treatment [see Warnings and Precautions (5.3)].
Post-dose elevated BP with life-threatening consequences
Discontinue ALIQOPA.
Non-infectious pneumonitis (NIP)
Grade 2
Withhold ALIQOPA and treat NIP. If NIP recovers to Grade 0 or 1, resume ALIQOPA at 45 mg.
If Grade 2 NIP recurs, discontinue ALIQOPA.
Grade 3 or higher
Discontinue ALIQOPA.
Neutropenia
Absolute neutrophil count (ANC) 0.5 to 1.0 x 103 cells/mm3
Maintain ALIQOPA dose. Monitor ANC at least weekly.
ANC less than 0.5 x 103 cells/mm3
Withhold ALIQOPA. Monitor ANC at least weekly until ANC 0.5 x 103 cells/mm3 or greater, then resume ALIQOPA at previous dose. If ANC 0.5 x 103 cells/mm3 or less recurs, then reduce ALIQOPA to 45 mg.
Severe cutaneous reactions
Grade 3
Withhold ALIQOPA until toxicity is resolved and reduce ALIQOPA from 60 mg to 45 mg or from 45 mg to 30 mg.
Life-threatening
Discontinue ALIQOPA.
Thrombocytopenia
Less than 25 x 109/L
Withhold ALIQOPA; resume when platelet levels return to 75.0 x 109/L or greater. If recovery occurs within 21 days, reduce ALIQOPA from 60 mg to 45 mg or from 45 mg to 30 mg. If recovery does not occur within 21 days, discontinue ALIQOPA.
Other severe and non-life-threatening toxicities
Grade 3
Withhold ALIQOPA until toxicity is resolved and reduce ALIQOPA from 60 mg to 45 mg or from 45 mg to 30 mg.
aEnsure a minimum of 7 days between any two consecutive infusions.
bNational Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE) v4.03.
cBoth systolic of less than 150 mmHg and diastolic of less than 90 mmHg are required.
2.5 Preparation and Administration
For intravenous infusion only.
Administer ALIQOPA as a single agent, following reconstitution and dilution. Mix only with 0.9% sodium chloride (NaCl) solution. Do not mix or inject ALIQOPA with other drugs or other diluents.
2.6 Reconstitution Instructions
Reconstitute ALIQOPA with 4.4 mL of sterile 0.9% NaCl solution leading to a concentration of 15 mg/mL.
- Withdraw 4.4 mL of sterile 0.9% NaCl solution by using a 5 mL sterile syringe with needle.
- Inject the measured volume through the disinfected stopper surface into the vial of ALIQOPA.
- Dissolve the lyophilized solid by gently shaking the injection vial for 30 seconds.
- Allow to stand for one minute to let bubbles rise to the surface.
- Check if any undissolved substance is still seen. If yes, repeat the gentle shaking and settling procedure.
- Inspect visually for discoloration and particulate matter. After reconstitution, the solution should be colorless to slightly yellowish.
- Once the solution is free of visible particles, withdraw the reconstituted solution for further dilution.
2.7 Dilution Instructions for Intravenous Use
Further dilute the reconstituted solution in 100 mL sterile 0.9% NaCl solution for injection. With a sterile syringe, withdraw the required amount of the reconstituted solution for the desired dosage:
60 mg: Withdraw 4 mL of the reconstituted solution with a sterile syringe.
45 mg: Withdraw 3 mL of the reconstituted solution with a sterile syringe.
30 mg: Withdraw 2 mL of the reconstituted solution with a sterile syringe.
Inject the contents of the syringe into the patient infusion bag of 100 mL sterile 0.9% NaCl solution. Mix the dose well by inverting.
Discard any unused reconstituted or diluted solution appropriately.
Use reconstituted and diluted ALIQOPA immediately or store the reconstituted solution in the vial or diluted solution in the infusion bag at 2°C to 8°C (36°F to 46°F) for up to 24 hours before use. Allow the product to adapt to room temperature before use following refrigeration. Avoid exposure of the diluted solution to direct sunlight.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Infections
Serious, including fatal, infections occurred in 19% of 317 patients treated with ALIQOPA monotherapy. The most common serious infection was pneumonia [see Adverse Reactions (6.1)]. Monitor patients for signs and symptoms of infection and withhold ALIQOPA for Grade 3 and higher infection [see Dosage and Administration (2.4)].
Serious pneumocystis jiroveci pneumonia (PJP) occurred in 0.6% of 317 patients treated with ALIQOPA monotherapy [see Adverse Reactions (6.1)]. Before initiating treatment with ALIQOPA, consider PJP prophylaxis for populations at risk. Withhold ALIQOPA in patients with suspected PJP infection of any grade. If confirmed, treat infection until resolution, then resume ALIQOPA at previous dose with concomitant PJP prophylaxis [see Dosage and Administration (2.4)].
5.2 Hyperglycemia
Grade 3 or 4 hyperglycemia (blood glucose 250 mg/dL or greater) occurred in 41% of 317 patients treated with ALIQOPA monotherapy [see Adverse Reactions (6.1)]. Serious hyperglycemic events occurred in 2.8% of patients. Treatment with ALIQOPA may result in infusion-related hyperglycemia. Blood glucose levels typically peaked 5 to 8 hours post-infusion and subsequently declined to baseline levels for a majority of patients; blood glucose levels remained elevated in 17.7% of patients one day after ALIQOPA infusion. Of 155 patients with baseline HbA1c <5.7%, 16 (10%) patients had HbA1c >6.5% at the end of treatment.
Of the twenty patients with diabetes mellitus treated in CHRONOS-1, seven developed Grade 4 hyperglycemia and two discontinued treatment. Patients with diabetes mellitus should only be treated with ALIQOPA following adequate glucose control and should be monitored closely.
Achieve optimal blood glucose control before starting each ALIQOPA infusion. Withhold, reduce dose, or discontinue ALIQOPA depending on the severity and persistence of hyperglycemia [see Dosage and Administration (2.4)].
5.3 Hypertension
Grade 3 hypertension (systolic 160 mmHg or greater or diastolic 100 mmHg or greater) occurred in 26% of 317 patients treated with ALIQOPA monotherapy [see Adverse Reactions (6.1)]. Serious hypertensive events occurred in 0.9% of 317 patients. Treatment with ALIQOPA may result in infusion-related hypertension. The mean change of systolic and diastolic BP from baseline to 2 hours post-infusion on Cycle 1 Day 1 was 16.8 mmHg and 7.8 mmHg, respectively. The mean BP started decreasing approximately 2 hours post-infusion; BP remained elevated for 6 to 8 hours after the start of the ALIQOPA infusion. Optimal BP control should be achieved before starting each ALIQOPA infusion. Monitor BP pre- and post-infusion. Withhold, reduce dose, or discontinue ALIQOPA depending on the severity and persistence of hypertension [see Dosage and Administration (2.4)].
5.4 Non-Infectious Pneumonitis
Non-infectious pneumonitis occurred in 5% of 317 patients treated with ALIQOPA monotherapy [see Adverse Reactions (6.1)]. Withhold ALIQOPA and conduct a diagnostic examination of a patient who is experiencing pulmonary symptoms such as cough, dyspnea, hypoxia, or interstitial infiltrates on radiologic exam. Patients with pneumonitis thought to be caused by ALIQOPA have been managed by withholding ALIQOPA and administration of systemic corticosteroids. Withhold, reduce dose, or discontinue ALIQOPA depending on the severity and persistence of non-infectious pneumonitis [see Dosage and Administration (2.4)].
5.5 Neutropenia
Grade 3 or 4 neutropenia occurred in 24% of 317 patients treated with ALIQOPA monotherapy. Serious neutropenic events occurred in 1.3% [see Adverse Reactions (6.1)]. Monitor blood counts at least weekly during treatment with ALIQOPA. Withhold, reduce dose, or discontinue ALIQOPA depending on the severity and persistence of neutropenia [see Dosage and Administration (2.4)].
5.6 Severe Cutaneous Reactions
Grade 3 and 4 cutaneous reactions occurred in 2.8% and 0.6% of 317 patients treated with ALIQOPA monotherapy, respectively [see Adverse Reactions (6.1)]. Serious cutaneous reaction events were reported in 0.9%. The reported events included dermatitis exfoliative, exfoliative rash, pruritus, and rash (including maculo-papular rash). Withhold, reduce dose, or discontinue ALIQOPA depending on the severity and persistence of severe cutaneous reactions [see Dosage and Administration (2.4)].
5.7 Embryo-Fetal Toxicity
Based on findings in animals and its mechanism of action, ALIQOPA can cause fetal harm when administered to a pregnant woman. In animal reproduction studies, administration of copanlisib to pregnant rats during organogenesis caused embryo-fetal death and fetal abnormalities in rats at maternal doses as low as 0.75 mg/kg/day (4.5 mg/m2/day body surface area) corresponding to approximately 12% the recommended dose for patients. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential and males with female partners of reproductive potential to use effective contraception during treatment and for at least one month after the last dose [see Use in Specific Populations (8.1, 8.3) and Clinical Pharmacology (12.1)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling.
- Infections [see Warnings and Precautions (5.1)]
- Hyperglycemia [see Warnings and Precautions (5.2)]
- Hypertension [see Warnings and Precautions (5.3)]
- Non-infectious pneumonitis [see Warnings and Precautions (5.4)]
- Neutropenia [see Warnings and Precautions (5.5)]
- Severe cutaneous reactions [see Warnings and Precautions (5.6)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in the general patient population.
The safety data reflect exposure to ALIQOPA in 168 adults with follicular lymphoma and other hematologic malignancies treated with ALIQOPA 60 mg or 0.8 mg/kg equivalent in clinical trials. The median duration of treatment was 22 weeks (range 1 to 206 weeks).
Serious adverse reactions were reported in 44 (26%) patients. The most frequent serious adverse reactions that occurred were pneumonia (8%), pneumonitis (5%) and hyperglycemia (5%). The most common adverse reactions (≥20%) were hyperglycemia, diarrhea, decreased general strength and energy, hypertension, leukopenia, neutropenia, nausea, lower respiratory tract infections, and thrombocytopenia.
Adverse reactions resulted in dose reduction in 36 (21%) and discontinuation in 27 (16%) patients. The most common reasons for dose reduction were hyperglycemia (7%), neutropenia (5%), and hypertension (5%). The most common reasons for drug discontinuation were pneumonitis (2%) and hyperglycemia (2%).
Table 2 provides the adverse reactions occurring in at least 10% of patients receiving ALIQOPA monotherapy, and Table 3 provides the treatment-emergent laboratory abnormalities in ≥20% of patients and ≥4% of Grade ≥3 treated with ALIQOPA.
Table 2: Adverse Reactions Reported in ≥10% of Patients with Follicular Lymphoma and Other Hematological Malignancies Treated with ALIQOPA ADVERSE REACTIONS
ALIQOPA
N = 168
Any Grade
n (%)
Grade 3
n (%)
Grade 4
n (%)
Metabolism and nutrition disorders
- Hyperglycemia
90 (54%)
56 (33%)
10 (6%)
Blood and lymphatic system disorders
- Leukopenia
61 (36%)
20 (12%)
26 (15%)
- Neutropenia (including febrile neutropenia)
53 (32%)
16 (10%)
26 (15%)
- Thrombocytopenia
37 (22%)
12 (7%)
2 (1%)
General disorders and administration site conditions
- Decreased general strength and energy (includes fatigue and asthenia)
61 (36%)
6 (4%)
0
Gastrointestinal disorders
- Diarrhea
60 (36%)
8 (5%)
0
- Nausea
43 (26%)
1 (<1%)
0
- Stomatitis (includes oropharyngeal erosion and ulcer, oral pain)
24 (14%)
3 (2%)
0
- Vomiting
21 (13%)
0
0
Vascular disorders
- Hypertension (includes secondary hypertension)
59 (35%)
46 (27%)
0
Infections
- Lower respiratory tract infections (includes pneumonia, pneumonia bacterial, pneumonia pneumococcal, pneumonia fungal, pneumonia viral, pneumocystis jiroveci pneumonia, bronchopulmonary aspergillosis and lung infection)
35 (21%)
20 (12%)
3 (2%)
Skin and subcutaneous tissue disorders
- Rash (includes exfoliative skin reactions)
26 (15%)
2 (1%)
1 (<1%)
Additional adverse drug reactions reported at a frequency of <10% in patients with follicular lymphoma and other hematologic malignancies include pneumonitis (9%), mucosal inflammation (8%), and paresthesia and dysesthesia (7%).
Table 3: Treatment-emergent Laboratory Abnormalities in ≥20% of Patients and ≥4% of Grade ≥3 Treated with ALIQOPA Laboratory Parameter
ALIQOPA N = 168*
Any Grade**
n (%)
Grade 3**
n (%)
Grade 4**
n (%)
Hematology abnormalities
Decreased hemoglobin
Lymphocyte count decreased
White blood cell decreased
Platelet count decreased
Neutrophil count decreased
130 (78%)
126 (78%)
118 (71%)
109 (65%)
104 (63%)
7 (4%)
43 (27%)
30 (18%)
11 (7%)
20 (12%)
0
4 (2%)
3 (2%)
3 (2%)
25 (15%)
Serum chemistry abnormalities
Hyperglycemia
Hypertriglyceridemia
Hypophosphatemia
Hyperuricemia
Serum lipase increased
160 (95%)
74 (58%)
72 (44%)
42 (25%)
34 (21%)
72 (43%)
6 (5%)
24 (15%)
40 (24%)
11 (7%)
9 (5%)
0
0
2 (1%)
2 (1%)
*Denominator for each laboratory parameter may vary based on number of patients with specific numeric laboratory values available.
**NCI-CTCAE v4.03
-
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on Copanlisib
Table 4 lists the potential effects of coadministration of ALIQOPA with strong CYP3A inhibitors and inducers.
Table 4: Drug Interactions with ALIQOPA that affect Copanlisib Concentrations Strong CYP3A inducers
Clinical impact
- Concomitant use of ALIQOPA with strong CYP3A inducers may decrease copanlisib AUC and Cmax[see Clinical Pharmacology (12.3)].
Prevention management
- Avoid concomitant use of ALIQOPA with strong CYP3A inducers
Strong CYP3A inhibitors
Clinical impact
- Concomitant use of ALIQOPA with strong CYP3A inhibitors increases the copanlisib AUC [see Clinical Pharmacology (12.3)].
- An increase in the copanlisib AUC may increase the risk of adverse reactions
Prevention management
- If concomitant use with strong CYP3A inhibitors cannot be avoided, reduce the ALIQOPA dose to 45 mg [see Dosage and Administration (2.3)]
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings from animal studies and the mechanism of action, ALIQOPA can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)].
There are no available data in pregnant women to inform the drug-associated risk. In animal reproduction studies, administration of copanlisib to pregnant rats during organogenesis resulted in embryo-fetal death and fetal abnormalities at maternal doses approximately 12% of the recommended dose for patients (see Data). Advise pregnant women of the potential risk to a fetus.
Adverse outcomes in pregnancy occur regardless of the health of the mother or the use of medications. The background risk of major birth defects and miscarriage for the indicated population are unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
In an embryo-fetal development study in rats, pregnant animals received intravenous doses of copanlisib of 0, 0.75, or 3 mg/kg/day during the period of organogenesis. Administration of copanlisib at the dose of 3 mg/kg/day resulted in maternal toxicity and no live fetuses. Copanlisib administration at the dose of 0.75 mg/kg/day was maternally toxic and resulted in embryo-fetal death (increased resorptions, increased post-implantation loss, and decreased numbers of fetuses/dam). The dose of 0.75 mg/kg/day also resulted in increased incidence of fetal gross external (domed head, malformed eyeballs or eyeholes), soft tissue (hydrocephalus internus, ventricular septal defects, major vessel malformations), and skeletal (dysplastic forelimb bones, malformed ribs and vertebrae, and pelvis shift) abnormalities. The dose of 0.75 mg/kg/day (4.5 mg/m2 body surface area) in rats is approximately 12% of the recommended dose for patients.
Following administration of radiolabeled copanlisib to pregnant rats approximately 1.5% of the radioactivity (copanlisib and metabolites) reached the fetal compartment.
8.2 Lactation
Risk Summary
There are no data on the presence of copanlisib and/or metabolites in human milk, the effects on the breastfed child, or on milk production. Following administration of radiolabeled copanlisib to lactating rats, approximately 2% of the radioactivity was secreted into milk; the milk to plasma ratio of radioactivity was 25-fold. Because of the potential for serious adverse reactions in a breastfed child from copanlisib, advise a lactating woman not to breastfeed during treatment with ALIQOPA and for at least 1 month after the last dose.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
ALIQOPA can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)]. Conduct pregnancy testing prior to initiation of ALIQOPA treatment.
Contraception
Infertility
There are no data on the effect of ALIQOPA on human fertility. Due to the mechanism of action of copanlisib, and findings in animal studies, adverse effects on reproduction, including fertility, are expected [see Nonclinical Toxicology (13.1)].
8.5 Geriatric Use
No dose adjustment is necessary in patients ≥65 years of age. Of 168 patients with follicular lymphoma and other hematologic malignancies treated with ALIQOPA, 48% were age 65 or older while 16% were age 75 or older. No clinically relevant differences in efficacy were observed between elderly and younger patients. In patients ≥65 years of age, 30% experienced serious adverse reactions and 21% experienced adverse reactions leading to discontinuation. In the patients <65 years of age, 23% experienced serious adverse reactions and 11% experienced adverse reactions leading to discontinuation.
8.6 Hepatic Impairment
Reduce ALIQOPA dose to 45 mg for patients with moderate hepatic impairment (Child-Pugh B) [see Dosage and Administration (2.2)]. No dose adjustment is required for patients with mild hepatic impairment (total bilirubin ≤1 × upper limit of normal [ULN] and aspartate aminotransferase [AST] > ULN, or total bilirubin >1 to 1.5 × ULN and any AST). ALIQOPA has not been studied in subjects with severe hepatic impairment (Child-Pugh C) [see Clinical Pharmacology (12.3)].
-
11 DESCRIPTION
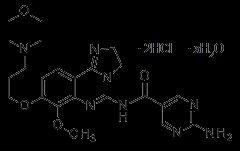
ALIQOPA (copanlisib) is a kinase inhibitor for intravenous infusion. The active pharmaceutical ingredient is copanlisib dihydrochloride which exists as a non-stoichiometric hydrate and has the molecular formula of C23H28N8O4 2HCl and a molecular weight of 553.45 g/mol. The molecular formula and molecular weight are based on the anhydrous form. The chemical name is 2-amino-N-{7-methoxy-8-[3-(morpholin-4-yl)propoxy]-2,3-dihydroimidazo[1,2-c]quinazolin-5-yl}pyrimidine-5-carboxamide dihydrochloride. Copanlisib dihydrochloride has the following structural formula:
ALIQOPA is supplied in single-dose vials as a sterile lyophilized solid for reconstitution and further dilution for intravenous infusion. The product is white to slightly yellowish. After reconstitution, the solution is colorless to slightly yellowish. Each vial contains 60 mg copanlisib free base (equivalent to 69.1 mg copanlisib dihydrochloride). After reconstitution, each mL contains 15 mg copanlisib free base (equivalent to 17.3 mg copanlisib dihydrochloride).
Inactive ingredients: Citric acid anhydrous, mannitol, sodium hydroxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Copanlisib is an inhibitor of phosphatidylinositol-3-kinase (PI3K) with inhibitory activity predominantly against PI3K-α and PI3K-δ isoforms expressed in malignant B cells. Copanlisib has been shown to induce tumor cell death by apoptosis and inhibition of proliferation of primary malignant B cell lines. Copanlisib inhibits several key cell-signaling pathways, including B-cell receptor (BCR) signaling, CXCR12 mediated chemotaxis of malignant B cells, and NFκB signaling in lymphoma cell lines.
12.2 Pharmacodynamics
At 60 mg (or 0.8 mg/kg) of ALIQOPA dose, the elevation of plasma glucose was associated with higher copanlisib exposure.
12.3 Pharmacokinetics
The area under the plasma concentration-time curve (AUC) and maximum plasma concentration (Cmax) of ALIQOPA increase dose-proportionally over 5 to 93 mg (0.08 to 1.55 times the approved recommended dose) absolute dose range and exhibit linear pharmacokinetics (PK). There is no time-dependency and no accumulation in the PK of copanlisib.
The geometric mean (range) steady state copanlisib exposure at 0.8 mg/kg (approximately the approved recommended dose of 60 mg) are 463 (range: 105 to 1670; SD: 584) ng/mL for Cmax and 1570 (range: 536 to 3410; SD: 338) ng.hr/mL for AUC0-25h.
Distribution
The in vitro human plasma protein binding of copanlisib is 84.2%. Albumin is the main binding protein. The in vitro mean blood-to-plasma ratio is 1.7 (range: 1.5 to 2.1). The geometric mean volume of distribution is 871 (range: 423 to 2150; SD: 479) L.
Elimination
The geometric mean terminal elimination half-life of copanlisib is 39.1 (range: 14.6 to 82.4; SD: 15.0) hours. The geometric mean clearance is 17.9 (range: 7.3 to 51.4; SD: 8.5) L/hr.
Metabolism
Approximately >90% of copanlisib metabolism is mediated by CYP3A and <10% by CYP1A1. The M-1 metabolite accounts for 5% of total radioactivity AUC and its pharmacological activity is comparable to the parent compound copanlisib for the tested kinases PI3KandPI3K.
Excretion
Copanlisib is excreted approximately 50% as unchanged compound and 50% as metabolites in humans. Following a single intravenous dose of 12 mg (0.2 times the recommended approved dose) radiolabeled copanlisib, approximately 64% of the administered dose was recovered in feces and 22% in urine within 20 to 34 days. Unchanged copanlisib represented approximately 30% of the administered dose in feces and 15% in urine. Metabolites resulting from CYP450-mediated oxidation metabolism accounted for 41% of the administered dose.
Specific Populations
Age (20 to 90 years), gender, race (White, Asian, Hispanic, and Black), smoking status, body weight (41 to 130 kg), and mild, moderate, and severe renal impairment [CLcr ≥ 15 mL/min as estimated by Cockcroft-Gault (C-G) equation] had no clinically significant effect on the PK of copanlisib. The PK of copanlisib in patients with end stage renal disease (CLcr < 15 mL/min by C-G equation) with or without dialysis is unknown.
Patients with Hepatic Impairment
Based on a population PK analysis in patients with cancer, mild hepatic impairment [total bilirubin ≤ 1 x ULN and AST > ULN, or total bilirubin < 1-1.5 x ULN and any AST] had no clinically relevant effect on the PK of copanlisib.
In a dedicated PK study evaluating a single intravenous dose of 12 mg (0.2 times the recommended approved dose of 60 mg) of ALIQOPA in subjects with hepatic impairment, the geometric mean of total copanlisib Cmax and AUC increased 1.38-fold and 1.71-fold, respectively, in subjects with moderate hepatic impairment (Child-Pugh B) as compared to normal hepatic function. The geometric mean unbound AUC of copanlisib was increased by 1.23-fold with no effect on Cmax. The PK of copanlisib in patients with severe hepatic impairment (Child-Pugh C or total bilirubin = 3-10 x ULN and any AST) is unknown.
Drug Interaction Studies
Clinical Studies
Effect of CYP3A and P-gp Inducers on Copanlisib
Rifampin, a strong CYP3A and a P-glycoprotein (P-gp) transporter inducer, administered at a dose of 600 mg once daily for 12 days with a single intravenous dose of 60 mg ALIQOPA in patients with cancer resulted in a 60% decrease in the mean AUC and a 12% decrease in Cmax of copanlisib [see Drug Interactions (7.1)].
Effect of CYP3A, P-gp and BCRP Inhibitors on Copanlisib
Itraconazole, a strong CYP3A inhibitor and a P-gp and Breast Cancer Resistance Protein (BCRP) transporter inhibitor, administered at a dose of 200 mg once daily for 10 days increased the mean AUC of a single intravenous dose of 60 mg ALIQOPA by 53% (or 1.53-fold) with no effect on Cmax (1.03-fold) in patients with cancer [see Drug Interactions (7.1)].
In Vitro Studies
Effect of Transporters on Copanlisib:
Copanlisib is a substrate of P-gp and BCRP, but not a substrate for organic cation transporter (OCT) 1, OCT2, and OCT3, organic anion transporter (OAT) 1 and OAT3, organic anion-transporting polypeptide (OATP) 1B1 and OATP1B3, multidrug and toxin extrusion protein 1(MATE1) or MATE2-K.
Effect of Copanlisib on CYP and non-CYP Enzymes
Copanlisib is not an inhibitor of the metabolism of drugs that are substrates of the major CYP isoforms (CYP1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, and 3A4) or uridine diphosphate-glucuronosyltransferase isoforms (UGT) or dihydropyrimidine dehydrogenase (DPD) at therapeutic 60 mg dose plasma concentrations. Copanlisib is not an inducer of CYP1A2, CYP2B6 and CYP3A.
Effect of Copanlisib on Drug Transporter Substrates
Copanlisib is not an inhibitor of P-gp, BCRP, multi-drug resistance-associated protein (MRP2), bile salt export pump (BSEP), OATP1B1, OATP1B3, OAT1, OAT3, OCT1, OCT2, and MATE1 at therapeutic 60 mg dose plasma concentrations.
Copanlisib is an inhibitor of MATE2-K (IC50: 0.09 μM). Inhibition may occur after copanlisib infusion at approved recommended dosage. The clinical significance of this potential inhibition on plasma concentrations of concomitantly administered drugs that are MATE2-K substrates is unknown.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been conducted with copanlisib.
Copanlisib did not cause genetic damage in in vitro or in vivo assays.
Fertility studies with copanlisib were not conducted; however, adverse findings in male and female reproductive systems were observed in the repeat dose toxicity studies. Findings in the male rats and/or dogs included effects on the testes (germinal epithelial degeneration, decreased weight, and/or tubular atrophy), epididymides (spermatic debris, decreased weight, and/or oligospermia/aspermia), and prostate (reduced secretion and/or decreased weight). Findings in female rats included effects on ovaries (hemorrhage, hemorrhagic cysts, and decreased weight), uterus (atrophy, decreased weight), vagina (mononuclear infiltration), and a dose-related reduction in the numbers of female rats in estrus.
-
14 CLINICAL STUDIES
14.1 Relapsed Follicular Lymphoma
The efficacy of ALIQOPA was evaluated in a single-arm, multicenter, phase 2 clinical trial (NCT 01660451) CHRONOS-1 in a total of 142 subjects, which included 104 subjects with follicular B-cell non-Hodgkin lymphoma who had relapsed disease following at least two prior treatments. Patients must have received rituximab and an alkylating agent. Baseline patient characteristics are summarized in Table 5. The most common prior systemic therapies were chemotherapy in combination with anti-CD20 immunotherapy (89%), chemotherapy alone (41%), and anti-CD20 immunotherapy alone (37%). In CHRONOS-1, 34% of patients received two prior lines of therapy and 36% received three prior lines of therapy.
Table 5: Baseline Patient Characteristics (Follicular Lymphoma) Characteristics
ALIQOPA
N=104Age, years; median (range)
62 (25 to 81)
Caucasian
83%
Male
52%
ECOG performance status (0 or 1)
96%
Number of prior therapies; median (range)
3 (2 to 8)
Time since diagnosis, years; median (range)
5.8 (0.75 to 33.9)
Percent of patients refractory* to:
last regimen
62%
last anti-CD20 immunotherapy
57%
last alkylating agent
last combination anti-CD20 immunotherapy and alkylating agent
38%
41%
*Refractory: No response or progression of disease within six months of last treatment.
One hundred forty-two patients received 60 mg ALIQOPA; 130 patients received fixed dose 60 mg ALIQOPA and 12 patients received 0.8 mg/kg equivalent ALIQOPA administered as a 1-hour intravenous infusion on Days 1, 8, and 15 of a 28-day treatment cycle on an intermittent schedule (three weeks on and one week off). Treatment continued until disease progression or unacceptable toxicity. Tumor response was assessed according to the International Working Group response criteria for malignant lymphoma. Efficacy based on overall response rate (ORR) was assessed by an Independent Review Committee. Efficacy results from CHRONOS-1 are summarized in Table 6.
Table 6: Overall Response Rate (ORR) and Duration of Response (DOR) in Patients with Relapsed Follicular Lymphoma ALIQOPA
N=104
ORR, n (%)
61 (59%)
(95% CI)
(49, 68)
CR, n (%)
15 (14%)
PR, n (%)
46 (44%)
Median* DOR, months (range)
12.2 (0+, 22.6)
ORR = overall response rate; CI = confidence interval; CR = complete response;
PR = partial response; DOR = duration of response
*Kaplan-Meier estimate
The median time to response was 1.7 months (range 1.3 to 9.7 months).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
ALIQOPA is contained in a colorless glass vial closed with bromobutyl stopper with a flanged closure. Each vial of ALIQOPA contains copanlisib as a lyophilized solid.
NDC
Strength
Reconstituted Concentration
50419-385-01
60 mg
(one single-dose vial per carton)15 mg/mL
16.2 Storage and Handling
Product after reconstitution
Administer reconstituted and diluted solution immediately. If not, refrigerate at 2°C to 8°C (36°F to 46°F) and use within 24 hours. After refrigeration, allow the product to adapt to room temperature before use. Avoid exposure of the diluted solution to direct sunlight.
Mix only with 0.9% NaCl solution. Do not mix or inject ALIQOPA with other drugs or other diluents [see Dosage and Administration (2.5)].
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
- Infections – Advise patients that ALIQOPA can cause serious infections that may be fatal. Advise patients to immediately report symptoms of infection [see Warnings and Precautions (5.1)].
- Hyperglycemia – Advise patients that an infusion-related increase in blood glucose may occur, and to notify their healthcare provider of any symptoms such as pronounced hunger, excessive thirst, headaches, or frequently urinating. Blood glucose levels should be well controlled prior to infusion [see Warnings and Precautions (5.2)].
- Hypertension – Advise patients that an infusion-related increase in blood pressure may occur, and to notify their healthcare provider of any symptoms such as dizziness, passing out, headache, and/or a pounding heart. Blood pressure should be normal or well controlled prior to infusion [see Warnings and Precautions (5.3)].
- Non-infectious pneumonitis – Advise patients of the possibility of pneumonitis, and to report any new or worsening respiratory symptoms including cough or difficulty breathing [see Warnings and Precautions (5.4)].
- Neutropenia – Advise patients of the need for periodic monitoring of blood counts and to notify their healthcare provider immediately if they develop a fever or any signs of infection [see Warnings and Precautions (5.5)].
- Severe cutaneous reactions – Advise patients that a severe cutaneous reaction may occur, and to notify their healthcare provider if they develop skin reactions (rash, redness, swelling, itching or peeling of the skin) [see Warnings and Precautions (5.6)].
- Pregnancy – Advise females of reproductive potential to use effective contraceptive methods and to avoid becoming pregnant during treatment with ALIQOPA and for at least one month after the last dose. Advise patients to notify their healthcare provider immediately in the event of a pregnancy or if pregnancy is suspected during ALIQOPA treatment. Advise males with female partners of reproductive potential to use effective contraception during treatment with ALIQOPA and for at least one month after the last dose [see Warnings and Precautions (5.7)].
- Lactation – Advise not to breastfeed during treatment with ALIQOPA and for at least 1 month after the last dose [see Use in Specific Populations (8.2)].
© 2017 Bayer HealthCare Pharmaceuticals Inc.
-
Patient Package Insert
PATIENT INFORMATION
ALIQOPA™ (AL-ih-KO-pah)
(copanlisib)
for injection
What is ALIQOPA?
ALIQOPA is a prescription medicine used to treat adults with follicular lymphoma (FL) when the disease has come back after treatment with at least two prior medicines.
It is not known if ALIQOPA is safe and effective in children.
Before receiving ALIQOPA, tell your healthcare provider about all of your medical conditions, including if you:
- have an infection
- have lung or breathing problems
- have high blood pressure (hypertension)
- have diabetes or high blood sugar (hyperglycemia)
-
are pregnant or plan to become pregnant. ALIQOPA can harm your unborn baby.
- o Your healthcare provider will perform a pregnancy test before starting treatment with ALIQOPA.
- o Females who are able to become pregnant should use effective birth control (contraception) during treatment with ALIQOPA and for at least 1 month after the last dose of ALIQOPA. Talk to your healthcare provider about birth control methods that may be right for you. Tell your healthcare provider right away if you become pregnant or think you are pregnant during treatment with ALIQOPA.
- o Males with female partners who are able to become pregnant should use effective birth control (contraception) during treatment with ALIQOPA and for at least 1 month after the last dose of ALIQOPA.
- are breastfeeding or plan to breastfeed. It is not known if ALIQOPA passes into your breast milk. Do not breastfeed during treatment with ALIQOPA and for at least 1 month after the last dose of ALIQOPA. Talk to your healthcare provider about the best way to feed your child during treatment with ALIQOPA.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Certain other medicines may affect how ALIQOPA works.
Know the medicines you take. Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new medicine.
How will I receive ALIQOPA?
- ALIQOPA will be given to you by a healthcare provider as an intravenous (IV) injection into your vein over 1 hour.
- You will receive your ALIQOPA treatment 1 time every week for 3 weeks and then stop for 1 week. This is 1 cycle of treatment. ALIQOPA is usually given on Day 1, Day 8, and Day 15 of a 28-day treatment cycle.
- Your healthcare provider will decide how many treatment cycles you need.
- Your healthcare provider may withhold treatment, decrease your dose, temporarily stop, or permanently stop treatment with ALIQOPA if you have certain side effects.
What should I avoid while receiving ALIQOPA?
- Avoid taking St. John’s Wort during treatment with ALIQOPA.
- Avoid drinking grapefruit juice during treatment with ALIQOPA.
What are the possible side effects of ALIQOPA?
ALIQOPA can cause serious side effects, including:
- Infections. ALIQOPA can cause serious infections that may lead to death. The most common serious infection was pneumonia. Tell your healthcare provider right away if you have a fever or any signs of an infection during treatment with ALIQOPA.
- High blood sugar (hyperglycemia). High blood sugar is common following ALIQOPA infusion and can sometimes be serious. Tell your healthcare provider if you develop any symptoms of hyperglycemia during treatment with ALIQOPA. Symptoms of hyperglycemia may include:
- o being very hungry
- o headaches
- o being very thirsty
- o frequent urination
- High blood pressure (hypertension). High blood pressure is common following ALIQOPA infusion and can sometimes be serious.
- Lung or breathing problems. Your healthcare provider may do tests to check your lungs if you have breathing problems during treatment with ALIQOPA. Tell your healthcare provider right away if you develop new or worsening cough, shortness of breath, or difficulty breathing.
- Low white blood cell count (neutropenia). Neutropenia is common with ALIQOPA treatment and can sometimes be serious. Your healthcare provider will check your blood counts regularly during treatment with ALIQOPA. Tell your healthcare provider right away if you have a fever or any signs of infection during treatment with ALIQOPA.
- Severe skin reactions. Skin peeling, rash, and itching are common with ALIQOPA and can sometimes be serious. Tell your healthcare provider if you develop skin peeling, itching, or rash during treatment with ALIQOPA. Your healthcare provider may withhold treatment, decrease your dose, or permanently stop treatment if you develop severe skin reactions during treatment with ALIQOPA.
The most common side effects of ALIQOPA include:
- low white blood cell count (leukopenia)
- low platelets in your blood (thrombocytopenia)
- diarrhea
- decreased strength and tiredness
- lower respiratory tract infection
- nausea
These are not all of the possible side effects of ALIQOPA. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of ALIQOPA
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your healthcare provider or pharmacist for information about ALIQOPA that is written for health professionals.
What are the ingredients in ALIQOPA?
Active ingredient: copanlisib
Inactive ingredients: citric acid anhydrous, mannitol, sodium hydroxide
Manufactured in Germany
Manufactured for: Bayer HealthCare Pharmaceuticals Inc., Whippany, NJ 07981 USA
© 2017 Bayer HealthCare Pharmaceuticals Inc.
For more information, go to www.aliqopa.com or call 1-888-842-2937.
This Patient Information has been approved by the U.S. Food and Drug Administration Issued: September 2017
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC: 50419-385-01
Rx only
Aliqopa (copanlisib)
60 mg*
*Equivalent to 69.1 mg copanlisib dihydrochloride
For intravenous infusion only.
Must be reconstituted and diluted.
Single dose vial - discard unused portion
-
INGREDIENTS AND APPEARANCE
ALIQOPA
copanlisib injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 50419-385 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COPANLISIB (UNII: WI6V529FZ9) (COPANLISIB - UNII:WI6V529FZ9) COPANLISIB 15 mg in 1 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) MANNITOL (UNII: 3OWL53L36A) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50419-385-01 1 in 1 CARTON 09/14/2017 1 4 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product 2 NDC: 50419-385-72 1 in 1 CARTON 10/30/2018 2 4 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA209936 09/14/2017 Labeler - Bayer HealthCare Pharmaceuticals Inc. (005436809) Establishment Name Address ID/FEI Business Operations Sharp Corporation 143696495 REPACK(50419-385) Establishment Name Address ID/FEI Business Operations Bayer AG 323208116 ANALYSIS(50419-385) , API MANUFACTURE(50419-385) Establishment Name Address ID/FEI Business Operations Bayer AG 315097875 ANALYSIS(50419-385) , API MANUFACTURE(50419-385) , LABEL(50419-385) , MANUFACTURE(50419-385) , PACK(50419-385) , STERILIZE(50419-385)
Trademark Results [ALIQOPA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ALIQOPA 86796421 5144321 Live/Registered |
Bayer Intellectual Property GmbH 2015-10-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.