Acetaminophen by Solupharm Pharmazeutische Erzeugnisse GmbH ACETAMINOPHEN injection
Acetaminophen by
Drug Labeling and Warnings
Acetaminophen by is a Prescription medication manufactured, distributed, or labeled by Solupharm Pharmazeutische Erzeugnisse GmbH. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use, see full prescribing information for and Initial U.S. Approval. See full
prescribing information for ACETAMINOPHEN injection for intravenous use.
ACETAMINOPHEN Injection for intravenous use
Initial U.S. Approval: 1951RECENT MAJOR CHANGES
Dosage and Administration (2.5) 11/2016
Dosage and Administration (2.4) 01/2017INDICATIONS AND USAGE
ACETAMINOPHEN injection for intravenous use is indicated for the
Management of mild to moderate pain in adult and pediatric patients 2 years and older (1)
Management of moderate to severe pain with adjunctive opioid analgesics in adult and pediatric patients 2 years and older (1)
Reduction of fever in adult and pediatric patients (1) (1)DOSAGE AND ADMINISTRATION
ACETAMINOPHEN injection for intravenous use may be given as a single or repeated dose. (2.1)
ACETAMINOPHEN injection for intravenous use should be administered only as a 15-minute intravenous infusion. (2.4)
Adults and Adolescents Weighing 50 kg and Over:
1000 mg every 6 hours or 650 mg every 4 hours to a maximum of 4000 mg per day. Minimum dosing interval of 4 hours. (2.2)
Adults and Adolescents Weighing Under 50 kg:
15 mg/kg every 6 hours or 12.5 mg/kg every 4 hours to a maximum of 75 mg/kg per day. Minimum dosing interval of 4 hours. (2.2)
Children:
Children 2 to 12 years of age: 15 mg/kg every 6 hours or 12.5 mg/kg every 4 hours to a maximum of 75 mg/kg per day. Minimum dosing interval of 4 hours. (2.3)
Neonates and Infants:
Neonates including premature neonates born at ≥ 32 weeks gestational age to 28 days chronological age, 12.5 mg/kg every 6 hours to a maximum of 50 mg/kg per day. Minimum dosing interval of 6 hours. (2.4)
Infants (29 days to 2 years of age): 15 mg/kg every 6 hours to a maximum of 60 mg/kg per day. Minimum dosing interval of 6 hours. (2.4) (2) (2)DOSAGE FORMS AND STRENGTHS
Injection for intravenous infusion.
Each 100 mL glass vial contains 1000 mg acetaminophen (10 mg/mL). (3) (3)CONTRAINDICATIONS
Acetaminophen is contraindicated:
In patients with known hypersensitivity to acetaminophen or to any of the excipients in the IV formulation. (4)
In patients with severe hepatic impairment or severe active liver disease. (4) (4)WARNINGS AND PRECAUTIONS
Administration of acetaminophen in doses higher than recommended (by all routes of administration and from all acetaminophen-containing products including combination products) may result in hepatic injury, including the risk of liver failure and death. (5.1)
Use caution when administering acetaminophen in patients with the following conditions: hepatic impairment or active hepatic disease, in cases of alcoholism, chronic malnutrition, severe hypovolemia, or severe renal impairment (creatinine clearance ≤ 30 mL/min). (5.1)
Discontinue ACETAMINOPHEN injection for intravenous use immediately at the first appearance of skin rash and if symptoms associated with allergy or hypersensitivity occur. Do not use in patients with acetaminophen allergy. (5.2, 5.4)
Take care when prescribing, preparing, and administering ACETAMINOPHEN injection for intravenous use to avoid dosing errors which could result in accidental overdose and death. (5.3) (5)ADVERSE REACTIONS
The most common adverse reactions in patients treated with ACETAMINOPHEN injection for intravenous use were nausea, vomiting, headache, and insomnia in adult patients; nausea, vomiting, constipation, and pruritus in pediatric patients. (6.1) (6) (6)
To report SUSPECTED ADVERSE REACTIONS, contact Hikma at us.hikma@ primevigilance.com or call 1-877-845-0689 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. (6) (6)
DRUG INTERACTIONS
Substances that induce or regulate hepatic cytochrome enzyme CYP2E1 may alter the metabolism of acetaminophen and increase its hepatotoxic potential. (7.1)
Chronic oral acetaminophen use at a dose of 4000 mg/day has been shown to cause an increase in international normalized ratio (INR) in some patients who have been stabilized on sodium warfarin as an anticoagulant. (7.2) (7) (7)USE IN SPECIFIC POPULATIONS
Pediatric Use: The effectiveness of ACETAMINOPHEN injection for intravenous use for the treatment of acute pain in pediatric patients younger than 2 years of age has not been established. The safety and effectiveness of ACETAMINOPHEN injection for intravenous use in pediatric patients is supported by evidence from adequate and well controlled studies in adults with additional safety and pharmacokinetic data for this age group. (8.4)
Geriatric Use: No overall differences in safety or effectiveness were observed between geriatric and younger subjects. (8.5)
Hepatic Impairment: ACETAMINOPHEN injection for intravenous use is contraindicated in patients with severe hepatic impairment or severe active liver disease and should be used with caution in patients with hepatic impairment or active liver disease. (4, 5.1, 8.6)
Renal Impairment: In cases of severe renal impairment, longer dosing intervals and a reduced total daily dose of acetaminophen may be warranted. (5.1, 8.7) (8) (8)Revised: 05/2022 (8) (8)
Revised: 2/2023 (8)
(8)
Revised: 2/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Information
2.2 Recommended Dosage: Adults and Adolescents
2.3 Recommended Dosage: Children
2.4 Recommended Dosage For Treatment of Fever in Neonates and Infants
2.5 Instructions for Intravenous Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hepatic Injury
5.2 Serious Skin Reactions
5.3 Risk of Medication Errors
5.4 Allergy and Hypersensitivity
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
7 DRUG INTERACTIONS
7.1 Effects of Other Substances on Acetaminophen
7.2 Anticoagulants
USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Patients with Hepatic Impairment
8.7 Patients with Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Adult Acute Pain
14.2 Adult Fever
14.3 Pediatric Acute Pain and Fever
16 HOW SUPPLIED/STORAGE AND HANDLING
- * Sections or subsections omitted from the full prescribing information are not listed.
-
INDICATIONS & USAGE
1 INDICATIONS AND USAGE
Acetaminophen injection is indicated for
the management of mild to moderate pain in adult and pediatric patients 2 years and older
the management of moderate to severe pain with adjunctive opioid analgesics in adult and pediatric patients 2 years and older
the reduction of fever in adult and pediatric patients. -
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Information
Acetaminophen injection may be given as a single or repeated dose for the treatment of acute pain or fever. No dose adjustment is required when converting between oral acetaminophen and acetaminophen injection dosing in adults and adolescents who weigh 50 kg and above. Calculated maximum daily dose of acetaminophen is based on all routes of administration (i.e., intravenous, oral, and rectal) and all products containing acetaminophen. Exceeding the maximum mg/kg daily dose of acetaminophen as described in Tables 1-3 may result in hepatic injury, including the risk of liver failure and death. To avoid the risk of overdose, ensure that the total amount of acetaminophen from all routes and from all sources does not exceed the maximum recommended dose.
2.2 Recommended Dosage: Adults and Adolescents
Adults and adolescents weighing 50 kg and over: the recommended dosage of acetaminophen injection is 1000 mg every 6 hours or 650 mg every 4 hours, with a maximum single dose of acetaminophen injection of 1000 mg, a minimum dosing interval of 4 hours, and a maximum daily dose of acetaminophen of 4000 mg per day (includes all routes of administration and all acetaminophen-containing products including combination products).
Adults and adolescents weighing under 50 kg: the recommended dosage of acetaminophen injection is 15 mg/kg every 6 hours or 12.5 mg/kg every 4 hours, with a maximum single dose of acetaminophen injection of 15 mg/kg, a minimum dosing interval of 4 hours, and a maximum daily dose of acetaminophen of 75 mg/kg per day (includes all routes of administration and all acetaminophen-containing products including combination products).
Table 1. Dosing for Adults and Adolescents
Age group Dose given Dose given every 4 hours Dose given every 6 hours Maximum single dose Maximum total daily dose of acetaminophen (by all routes) Adults and
adolescents (13
years and older)
weighing ≥ 50 kg650 mg 1000 mg 1000 mg 4000 mg in 24 hours Adults and
adolescents (13
years and older)
weighing < 50 kg12.5 mg/kg 15 mg/kg 15 mg/kg
(up to 750 mg)75 mg/kg in 24 hours
(up to 3750 mg)2.3 Recommended Dosage: Children
Children 2 to 12 years of age: the recommended dosage of acetaminophen injection is 15 mg/kg every 6 hours or 12.5 mg/kg every 4 hours, with a maximum single dose of acetaminophen injection of 15 mg/kg, a minimum dosing interval of 4 hours, and a maximum daily dose of acetaminophen of 75 mg/kg per day. Table 2. Dosing for Children
Age group Dose given every 4 hours Dose given every 6 hours Maximum single dose Maximum total daily dose of acetaminophen (by all
routes)Children 2 to 12 years of Age 12.5 mg/kg 15 mg/kg 15 mg/kg (up to 750 mg) 75 mg/kg in 24 hours (up to 3750 mg) 2.4 Recommended Dosage For Treatment of Fever in Neonates and Infants
Neonates, including premature neonates born at 32 weeks gestational age, up to 28 days chronological age: the recommended dosage of acetaminophen injection is 12.5 mg/kg every 6 hours, to a maximum daily dose of acetaminophen of 50 mg/ kg per day, with a minimum dosing interval of 6 hours. Infants 29 days to 2 years of age: the recommended dosage of acetaminophen injection is 15 mg/kg every 6 hours, to a maximum daily dose of acetaminophen of 60 mg/kg per day, with a minimum dosing interval of 6 hours.
Table 3. Dosing for Treatment of Fever in Neonates and InfantsAge group Dose given every 6 hours Maximum total daily dose of acetaminophen (by all routes) Neonates (birth to 28 days) 12.5 mg/kg 50 mg/kg Infants (29 days to 2 years) 15 mg/kg 60 mg/kg 2.5 Instructions for Intravenous Administration
For adult and adolescent patients weighing ≥ 50 kg requiring 1000 mg doses of acetaminophen injection, administer the dose by inserting a vented intravenous set through the septum of the 100 mL vial. Acetaminophen injection may be administered without further dilution. Examine the vial contents before dose preparation or administering. DO NOT USE if particulate matter or discoloration is observed. Administer the contents of the vial intravenously over 15 minutes. Use aseptic technique when preparing acetaminophen injection for intravenous infusion. Do
not add other medications to the acetaminophen injection vial or infusion device.For doses less than 1000 mg, the appropriate dose must be withdrawn from the vial and placed into a separate container prior to administration. Using aseptic technique, withdraw the appropriate dose (650 mg or weight-based) from an intact sealed acetaminophen injection vial and place the measured dose in a separate empty, sterile container (e.g., glass bottle, plastic intravenous container, or syringe) for intravenous infusion to avoid the inadvertent delivery and administration of the total volume of the commercially available container. The entire 100 mL vial of acetaminophen injection is not intended for use in patients weighing less than 50 kg. Acetaminophen injection is supplied in a single-dose vial and the unused portion must be discarded.
Place small volume pediatric doses up to 60 mL in volume in a syringe and administer over 15 minutes using a syringe pump. Monitor the end of the infusion in order to prevent the possibility of an air embolism, especially in cases where the acetaminophen injection infusion is the primary infusion.
Once the vacuum seal of the glass vial has been penetrated, or the contents transferred to another container, administer the dose of acetaminophen injection within 6 hours.
Do not add other medications to the acetaminophen injection solution. Diazepam and chlorpromazine hydrochloride are physically incompatible with acetaminophen injection, therefore do not administer simultaneously. - 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hepatic Injury
Administration of acetaminophen in doses higher than recommended may result in hepatic injury, including the risk of liver failure and death [see Overdosage (10)]. Do not exceed the maximum recommended daily dose of acetaminophen [see Dosage and Administration (2)]. The maximum recommended daily dose of acetaminophen includes all routes of acetaminophen administration and all acetaminophen-containing products administered, including combination products.
Use caution when administering acetaminophen in patients with the following conditions: hepatic impairment or active hepatic disease, alcoholism, chronic malnutrition, severe hypovolemia (e.g., due to dehydration or blood loss), or severe renal impairment (creatinine clearance ≤ 30 mL/min) [see Use in Specific Populations (8.6, 8.7)].5.2 Serious Skin Reactions
Rarely, acetaminophen may cause serious skin reactions such as acute generalized exanthematous pustulosis (AGEP), Stevens-Johnson Syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal. Patients should be informed about the signs of serious skin reactions, and use of the drug should be discontinued at the first appearance of skin rash or any other sign of hypersensitivity.
5.3 Risk of Medication Errors
Take care when prescribing, preparing, and administering acetaminophen injection in order to avoid dosing errors which could result in accidental overdose and death. In particular, be careful to ensure that:
the dose in milligrams (mg) and milliliters (mL) is not confused;
the dosing is based on weight for patients under 50 kg;
infusion pumps are properly programmed; and
the total daily dose of acetaminophen from all sources does not exceed maximum daily limits [see Dosage and Administration (2)].5.4 Allergy and Hypersensitivity
There have been post-marketing reports of hypersensitivity and anaphylaxis associated with the use of acetaminophen. Clinical signs included swelling of the face, mouth, and throat, respiratory distress, urticaria, rash, and pruritus. There were infrequent reports of life-threatening anaphylaxis requiring emergent medical attention. Discontinue acetaminophen injection immediately if symptoms associated with allergy or hypersensitivity occur. Do not use acetaminophen injection in patients with acetaminophen allergy.
-
6 ADVERSE REACTIONS
ACETAMINOPHEN injection for intravenous use is indicated for the
Management of mild to moderate pain in adult and pediatric patients 2 years and older (1)
Management of moderate to severe pain with adjunctive opioid analgesics in adult and pediatric patients 2 years and older (1)
Reduction of fever in adult and pediatric patients (1) (1)6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed cannot be directly compared to rates in other clinical trials and may not reflect the rates observed in practice.
Adult Population
A total of 1020 adult patients have received acetaminophen injection in clinical trials, including 37.3% (n=380) who received 5 or more doses, and 17.0% (n=173) who received more than 10 doses. Most patients were treated with acetaminophen injection 1000 mg every 6 hours. A total of 13.1% (n=134) received acetaminophen injection 650 mg every 4 hours.All adverse reactions that occurred in adult patients treated with either acetaminophen injection or placebo in repeated dose, placebo-controlled clinical trials at an incidence 3% and at a greater frequency than placebo are listed in Table 4. The most common adverse events in adult patients treated with acetaminophen injection (incidence 5% and greater than placebo) were nausea, vomiting, headache, and insomnia.
Table 4. Treatment-Emergent Adverse Reactions Occurring in 3% of Acetaminophen Injection-treated Adult Patients and at a Greater Frequency than Placebo in Placebo-Controlled, Repeated Dose Studies
System Organ Class – Preferred
TermAcetaminophen Injection
(N=402)
n (%)Placebo
(N=379)
n (%)Gastrointestinal Disorders
Nausea
Vomiting138 (34)
62 (15)
119 (31)
42 (11)
General Disorders and Administration
Site Conditions
Pyrexia *22 (5) 52 (14) Nervous System Disorders
Headache39 (10) 33 (9) Psychiatric Disorders
Insomnia30 (7) 21 (5) * Pyrexia adverse reaction frequency data is included in order to alert healthcare practitioners that the antipyretic effects of Acetaminophen Injection may mask fever.
Other Adverse Reactions Observed During Clinical Studies of Acetaminophen Injection in Adults
The following additional treatment-emergent adverse reactions were reported by adult subjects treated with acetaminophen injection in all clinical trials (n=1020) that occurred with an incidence of at least 1% and at a frequency greater than placebo (n=525). Blood and lymphatic system disorders: anemia
General disorders and administration site conditions: fatigue, infusion site pain,
edema peripheral
I nvestigations: aspartate aminotransferase increased, breath sounds abnormal
Metabolism and nutrition disorders: hypokalemia
Musculoskeletal and connective tissue disorders: muscle spasms, trismus
Psychiatric disorders: anxiety
Respiratory, thoracic and mediastinal disorders: dyspnea
Vascular disorders: hypertension, hypotension
Pediatric PopulationA total of 483 pediatric patients (72 neonates, 167 infants, 171 children, and 73 adolescents) have received acetaminophen injection in active-controlled (n=250) and open-label clinical trials (n=225), including 43.9% (n=212) who received 5 or more doses and 31.2% (n=153) who received more than 10 doses. Pediatric patients received acetaminophen injection doses up to 15 mg/kg on an every 4 hours, every 6 hours, or every 8 hours schedule. The maximum exposure was 7.7, 6.4, 6.8, and 7.1 days in neonates, infants, children, and adolescents, respectively.
The most common adverse events (incidence ≥ 5%) in pediatric patients treated with acetaminophen injection were nausea, vomiting, constipation, and pruritus.
Other Adverse Reactions Observed During Clinical Studies of Acetaminophen Injection in Pediatrics
The following additional treatment-emergent adverse reactions were reported by pediatric subjects treated with acetaminophen injection (n=483) that occurred with an incidence of at least 1%.
Blood and lymphatic system disorders: anemia
Gastrointestinal disorders: diarrhea
General disorders and administration site conditions: pyrexia, injection site pain
Metabolism and nutrition disorders: hypokalemia, hypomagnesemia, hypoalbuminemia, hypophosphatemia
Musculoskeletal and connective tissue disorders: muscle spasm
Nervous system disorders: headache
Psychiatric disorders: agitation
Renal and urinary disorders: oliguria
Respiratory, thoracic and mediastinal disorders: atelectasis, pleural effusion, pulmonary edema, stridor, wheezing
Vascular disorders: hypotension, hypertension -
7 DRUG INTERACTIONS
7.1 Effects of Other Substances on Acetaminophen
Substances that induce or regulate hepatic cytochrome enzyme CYP2E1 may alter the metabolism of acetaminophen and increase its hepatotoxic potential. The clinical consequences of these effects have not been established. Effects of ethanol are complex, because excessive alcohol usage can induce hepatic cytochromes, but ethanol also acts as a competitive inhibitor of the metabolism of acetaminophen.
7.2 Anticoagulants
Chronic oral acetaminophen use at a dose of 4000 mg/day has been shown to cause an increase in international normalized ratio (INR) in some patients who have been stabilized on sodium warfarin as an anticoagulant. As no studies have been performed evaluating the short-term use of acetaminophen injection in patients on oral anticoagulants, more frequent assessment of INR may be appropriate in such circumstances.
-
USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Published epidemiological studies with oral acetaminophen use during pregnancy have not reported a clear association with acetaminophen use and birth defects, miscarriage, or adverse maternal or fetal outcomes [see Data]. Animal reproduction studies have not been conducted with IV acetaminophen. Reproductive and developmental studies in rats and mice from the published literature identified adverse events at clinically relevant doses with acetaminophen. Treatment of pregnant rats with doses of acetaminophen approximately equal to the maximum human
daily dose (MHDD) showed evidence of fetotoxicity and increases in bone variations in the fetuses. In another study, necrosis was observed in the liver and kidney of both pregnant rats and fetuses at doses approximately equal to the MHDD. In mice and rats treated with acetaminophen at doses within the clinical dosing range, cumulative adverse effects on reproductive capacity were reported. In mice, a reduction in number of litters of the parental mating pair was observed as well as retarded growth, abnormal sperm in their offspring and reduced birth weight in the
next generation. In rats, female fertility was decreased following in utero exposure to acetaminophen [see Data].The estimated background risk of major birth defects and miscarriages for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Human Data
The results from a large population-based prospective cohort, including data from 26,424 women with live born singletons who were exposed to oral acetaminophen during the first trimester, indicate no increased risk for congenital malformations, compared to a control group of unexposed children. The rate of congenital malformations (4.3%) was similar to the rate in the general population. A population-based, case-control study from the National Birth Defects Prevention Study showed that 11,610 children with prenatal exposure to acetaminophen during the first trimester
had no increased risk of major birth defects compared to 4,500 children in the control group. Other epidemiological data showed similar results. However, these studies cannot definitely establish the absence of any risk because of methodological limitations, including recall bias.
Animal Data
Studies in pregnant rats that received oral acetaminophen during organogenesis at doses up to 0.85 times the maximum human daily dose (MHDD = 4 grams/day, based on a body surface area comparison) showed evidence of fetotoxicity (reduced fetal weight and length) and a dose-related increase in bone variations (reduced ossification and rudimentary rib changes).Offspring had no evidence of external, visceral, or skeletal malformations. When pregnant rats received oral acetaminophen throughout gestation at doses of 1.2 times the MHDD (based on a body surface area comparison), areas of necrosis occurred in both the liver and kidney of pregnant rats and fetuses. These effects did not occur in animals that received oral acetaminophen at doses 0.3 times the MHDD, based on a body surface area comparison.
In a continuous breeding study, pregnant mice received 0.25, 0.5, or 1.0% acetaminophen via the diet (357, 715, or 1430 mg/kg/day). These doses are approximately 0.43, 0.87, and 1.7 times the MHDD, respectively, based on a body surface area comparison. A dose-related reduction in body weights of fourth and fifth litter offspring of the treated mating pair occurred during lactation and post-weaning at all doses. Animals in the high dose group had a reduced number of litters per mating pair, male offspring with an increased percentage of abnormal sperm, and reduced birth weights in the next generation pups.
8.2 Lactation
Risk Summary
There is no information regarding the presence of acetaminophen injection in human milk, the effects on the breastfed infant, or the effects on milk production. However, limited published studies report that acetaminophen passes rapidly into human milk with similar levels in the milk and plasma. Average and maximum neonatal doses of 1% and 2%, respectively, of the weight-adjusted maternal dose are reported after a single oral administration of 1 gram APAP. There is one well-documented report of a rash in a breast-fed infant that resolved when the mother stopped acetaminophen use and recurred when she resumed acetaminophen use. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for acetaminophen injection and any potential adverse effects on the breastfed infant from acetaminophen injection or from the underlying maternal condition.8.3 Females and Males of Reproductive Potential
Based on animal data use of acetaminophen may cause reduced fertility in males and females of reproductive potential. It is not known whether these effects on fertility are reversible. Published animal studies reported that oral acetaminophen treatment of male animals at doses that are 1.2 times the MHDD and greater (based on a body surface area comparison) result in decreased testicular weights, reduced spermatogenesis, and reduced fertility. In female animals given the same doses, reduced implantation sites were reported. Additional published animal studies indicate that acetaminophen exposure in utero adversely impacts reproductive capacity of both male and female offspring at clinically relevant exposures8.4 Pediatric Use
8.4 Pediatric Use
Treatment of Acute Pain
The safety and effectiveness of acetaminophen injection for the treatment of acute pain in pediatric patients ages 2 years and older is supported by evidence from adequate and well-controlled studies of acetaminophen injection in adults and safety and pharmacokinetic data from adult and 483 pediatric patients across all age groups [see Dosage and Administration (2.3) and Pharmacokinetics (12.3)].
The effectiveness of acetaminophen injection for the treatment of acute pain in pediatric patients younger than 2 years of age has not been established.
In patients younger than 2 years, efficacy was not demonstrated in a double-blind, placebo- controlled study of 198 pediatric patients younger than 2 years. Pediatric patients less than 2 years of age, including neonates from 28 to 40 weeks gestational age at birth, were randomized to receive opioid plus acetaminophen or opioid plus placebo. No difference in analgesic effect of intravenous acetaminophen, measured by assessment of reduced need for additional opioid treatment for pain control, was observed.
Treatment of Fever
The safety and effectiveness of acetaminophen injection for the treatment of fever in pediatric patients, including premature neonates born at ≥ 32 weeks gestational age is supported by adequate and well-controlled studies of acetaminophen injection in adults, clinical studies in 244 pediatric patients 2 years and older, and safety and pharmacokinetic data from 239 patients younger than 2 years including neonates ≥ 32 weeks gestational age.8.5 Geriatric Use
Of the total number of subjects in clinical studies of acetaminophen injection, 15% were age 65 and over, while 5% were age 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Patients with Hepatic Impairment
Acetaminophen is contraindicated in patients with severe hepatic impairment or severe active liver disease and should be used with caution in patients with hepatic impairment or active liver disease [see Warnings and Precautions (5.1) and Clinical Pharmacology (12)]. A reduced total daily dose of acetaminophen may be warranted.
-
10 OVERDOSAGE
Signs and Symptoms
In acute acetaminophen overdosage, dose-dependent, potentially fatal hepatic necrosis is the most serious adverse effect. Renal tubular necrosis, hypoglycemic coma, and thrombocytopenia may also occur. Plasma acetaminophen levels > 300 mcg/mL at 4 hours after oral ingestion were associated with hepatic damage in 90% of patients; minimal hepatic damage is anticipated if plasma levels at 4 hours are < 150 mcg/mL or < 37.5 mcg/mL at 12 hours after ingestion. Early symptoms following a potentially hepatotoxic overdose may include: nausea, vomiting, diaphoresis, and general malaise. Clinical and laboratory evidence of hepatic toxicity may not be apparent until 48 to 72 hours post-ingestion.Treatment
If an acetaminophen overdose is suspected, obtain a serum acetaminophen assay as soon as possible, but no sooner than 4 hours following oral ingestion. Obtain liver function studies initially and repeat at 24-hour intervals. Administer the antidote N-acetylcysteine (NAC) as early as possible. As a guide to treatment of acute ingestion, the acetaminophen level can be plotted against time since oral ingestion on a nomogram (Rumack-Matthew). The lower toxic line on the nomogram is equivalentto 150 mcg/mL at 4 hours and 37.5 mcg/mL at 12 hours. If serum level is
above the lower line, administer the entire course of NAC treatment. Withhold NAC therapy if the acetaminophen level is below the lower line.
For additional information, call a poison control center at 1-800-222-1222. -
11 DESCRIPTION
Acetaminophen is a non-salicylate antipyretic and non-opioid analgesic agent. Its chemical name is N-acetyl-p-aminophenol. Acetaminophen has a molecular weight of 151.16. Its structural formula is:
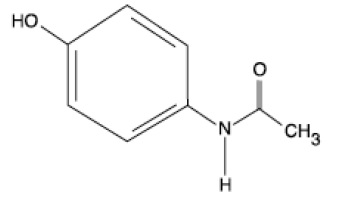
Acetaminophen injection is a sterile, clear, colorless to slightly yellowish, non pyrogenic, isotonic formulation of acetaminophen intended for intravenous infusion. It has a pH of approximately 5.5 and an osmolality of approximately 290 mOsm/kg. Each 100 mL contains 1000 mg acetaminophen, USP, 3850 mg D-mannitol, USP, 25 mg cysteine hydrochloride, monohydrate, USP, and 13 mg disodium phosphate dihydrate, USP. pH may have been adjusted with hydrochloric acid and/or sodium hydroxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The precise mechanism of the analgesic and antipyretic properties of acetaminophen is not established but is thought to primarily involve central actions.
12.2 Pharmacodynamics
Acetaminophen has been shown to have analgesic and antipyretic activities in animal and human studies.
Single doses of acetaminophen injection up to 3000 mg and repeated doses of 1000 mg every 6 hours for 48 hours have not been shown to cause a significant effect on platelet aggregation.
Acetaminophen does not have any immediate or delayed effects on small-vessel hemostasis. Clinical studies of both healthy subjects and patients with hemophilia showed no significant changes in bleeding time after receiving multiple doses of oral acetaminophen.12.3 Pharmacokinetics
Distribution
The pharmacokinetics of acetaminophen injection have been studied in patients and healthy subjects up to 60 years old. The pharmacokinetic profile of acetaminophen injection has been demonstrated to be dose proportional in adults following administration of single doses of 500, 650, and 1000 mg. The maximum concentration (Cmax) occurs at the end of the 15-minute intravenous infusion of acetaminophen injection. Compared to the same dose of oral acetaminophen, the Cmax following administration of acetaminophen injection is up to 70% higher, while overall exposure (area under the concentration time curve [AUC]) is very similar.Pharmacokinetic parameters of acetaminophen injection (AUC, Cmax, terminal elimination half-life [T½], systemic clearance [CL], and volume of distribution at steady state [Vss]) following administration of a single intravenous dose of 15 mg/ kg in children and adolescents and 1000 mg in adults are summarized in Table 5.
Table 5. Acetaminophen Injection Pharmacokinetic Parameters
Subpopulations AUC0-6h
(μg × h/
mL)Cmax
(μg/mL)T½
(h)CL
(L/h/kg)Vss (L/kg) Children 38 (8) 29 (7) 3.0 (1.5) 0.34 (0.10) 1.2 (0.3) Adolescents 41 (7) 31 (9) 2.9 (0.7) 0.29 (0.08) 1.1 (0.3) Adults 43 (11) 28 (21) 2.4 (0.6) 0.27 (0.08) 0.8 (0.2) The concentrations of acetaminophen injection observed in neonates greater than 32 weeks gestational age at birth treated with 12.5 mg/kg dose are similar to infants, children and adolescents treated with a 15 mg/kg dose, and similar to adults
treated with a 1000 mg dose.
At therapeutic levels, binding of acetaminophen to plasma proteins is low (ranging from 10% to 25%). Acetaminophen appears to be widely distributed throughout most body tissues except fat.Metabolism and Excretion
Acetaminophen is primarily metabolized in the liver by first-order kinetics and involves three principal separate pathways: Conjugation with glucuronide, conjugation with sulfate, and oxidation via the cytochrome P450 enzyme pathway, primarily CYP2E1, to form a reactive intermediate metabolite (N-acetyl-p-benzoquinone imine or NAPQI). With therapeutic doses, NAPQI undergoes rapid conjugation with glutathione and is then further metabolized to form cysteine and mercapturic acid conjugates.
Acetaminophen metabolites are mainly excreted in the urine. Less than 5% is excreted in the urine as unconjugated (free) acetaminophen and more than 90% of the administered dose is excreted within 24 hours. -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term studies in mice and rats have been completed by the National Toxicology Program to evaluate the carcinogenic potential of acetaminophen. In 2-year feeding studies, F344/N rats and B6C3F1 mice were fed a diet containing acetaminophen up to 6000 ppm. Female rats demonstrated equivocal evidence of carcinogenic activity based on increased incidences of mononuclear cell leukemia at 0.8 times the maximum human daily dose (MHDD) of 4 grams/day, based on a body surface area comparison. In contrast, there was no evidence of carcinogenic activity in male rats (0.7 times) or mice (1.2-1.4 times the MHDD, based on a body
surface area comparison).
Mutagenesis
Acetaminophen was not mutagenic in the bacterial reverse mutation assay (Ames test). In contrast, acetaminophen tested positive in the in vitro mouse lymphoma assay and the in vitro chromosomal aberration assay using human lymphocytes. In the published literature, acetaminophen has been reported to be clastogenic when administered a dose of 1500 mg/kg/day to the rat model (3.6 times the MHDD, based on a body surface area comparison). In contrast, no clastogenicity was noted at a dose of 750 mg/kg/day (1.8 times the MHDD, based on a body surface area comparison), suggesting a threshold effect.
Impairment of Fertility
In studies conducted by the National Toxicology Program, fertility assessments have been completed in Swiss mice via a continuous breeding study. There were no effects on fertility parameters in mice consuming up to 1.7 times the MHDD of acetaminophen, based on a body surface area comparison. Although there was no effect on sperm motility or sperm density in the epididymis, there was a significant increase in the percentage of abnormal sperm in mice consuming 1.7 times the MHDD (based on a body surface area comparison) and there was a reduction
in the number of mating pairs producing a fifth litter at this dose, suggesting the potential for cumulative toxicity with chronic administration of acetaminophen near the upper limit of daily dosing.
Published studies in rodents report that oral acetaminophen treatment of male animals at doses that are 1.2 times the MHDD and greater (based on a body surface area comparison) result in decreased testicular weights, reduced spermatogenesis, reduced fertility, and reduced implantation sites in females given the same doses. These effects appear to increase with the duration of treatment.
In a published mouse study, oral administration of 50 mg/kg acetaminophen to pregnant mice from Gestation Day 7 to delivery (0.06 times the MHDD, based on a body surface area comparison) reduced the number of primordial follicles in female offspring and reduced the percentage of full-term pregnancies and number of pups born to these females exposed to acetaminophen in utero.
In a published study, oral administration of 350 mg/kg acetaminophen to pregnant rats (0.85 times the MHDD, based on a body surface area comparison) from Gestation Day 13 to 21 (dams) reduced the number of germ cells in the fetal ovary, decreased ovary weight, and reduced the number of pups per litter in F1 females as well as reduced ovary weights in F2 females. -
14 CLINICAL STUDIES
14.1 Adult Acute Pain
The efficacy of acetaminophen injection in the treatment of acute pain in adults was evaluated in two randomized, double-blind, placebo-controlled clinical trials in patients with postoperative pain.
Pain Study 1 evaluated the analgesic efficacy of repeated doses of acetaminophen injection 1000 mg vs. placebo every 6 hours for 24 hours in 101 patients with moderate to severe pain following total hip or knee replacement. Acetaminophen injection was statistically superior to placebo for reduction in pain intensity over 24 hours. There was an attendant decrease in opioid consumption, the clinical benefit of which was not demonstrated.
Pain Study 2 evaluated the analgesic efficacy of repeated doses of acetaminophen injection 1000 mg every 6 hours or 650 mg every 4 hours for 24 hours versus placebo in the treatment of 244 patients with moderate to severe postoperative pain after abdominal laparoscopic surgery. Patients receiving acetaminophen injection experienced a statistically significant greater reduction in pain intensity over 24 hours compared to placebo.14.2 Adult Fever
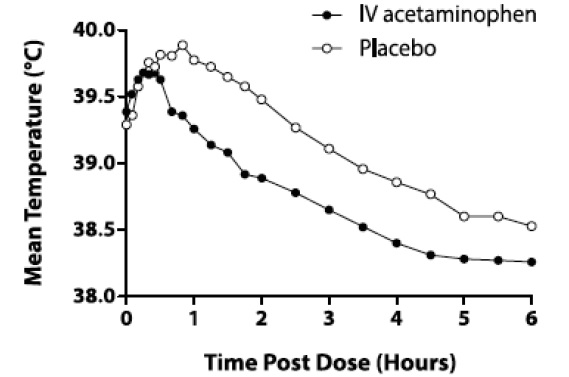
The efficacy of acetaminophen injection 1000 mg in the treatment of adult fever was evaluated in one randomized, double-blind, placebo-controlled clinical trial. The study was a 6-hour, single-dose, endotoxin-induced fever study in 60 healthy adult males. A statistically significant antipyretic effect of acetaminophen injection was demonstrated through 6 hours in comparison to placebo. The mean temperature over time is shown in Figure 1.
Figure 1: Mean Temperature (°C) Over Time
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Acetaminophen injection is supplied in a 100 mL glass vial containing 1000 mg acetaminophen (10 mg/mL) in cartons of 24 vials.
NDC: 24201-110-24
Acetaminophen injection should be stored at 20°C to 25°C (68°F to 77°F) [see USP Controlled Room Temperature].
For single-dose only. The product should be used within 6 hours after opening. Do not refrigerate or freeze.Manufactured for:
Hikma
Berkeley Heights, NJ 07922Made in Germany
Novaplus is a registered trademark of Vizient, Inc.
Revised: 05/2022
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
ACETAMINOPHEN
acetaminophen injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 55579-110 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 1000 mg in 100 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) CYSTEINE HYDROCHLORIDE (UNII: ZT934N0X4W) SODIUM PHOSPHATE, DIBASIC, DIHYDRATE (UNII: 94255I6E2T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 55579-110-01 24 in 1 BOTTLE 03/01/2023 1 100 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202605 12/07/2020 Labeler - Solupharm Pharmazeutische Erzeugnisse GmbH (316875129) Establishment Name Address ID/FEI Business Operations Solupharm Pharmazeutische Erzeugnisse GmbH 316875129 manufacture(55579-110)
Trademark Results [Acetaminophen]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ACETAMINOPHEN 85615223 not registered Dead/Abandoned |
General Merchandise importers and Expoters 2012-05-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.