Hand Sanitizer gel by Guangzhou Opseve Cosmetics Co., Ltd
Hand Sanitizer gel by
Drug Labeling and Warnings
Hand Sanitizer gel by is a Otc medication manufactured, distributed, or labeled by Guangzhou Opseve Cosmetics Co., Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
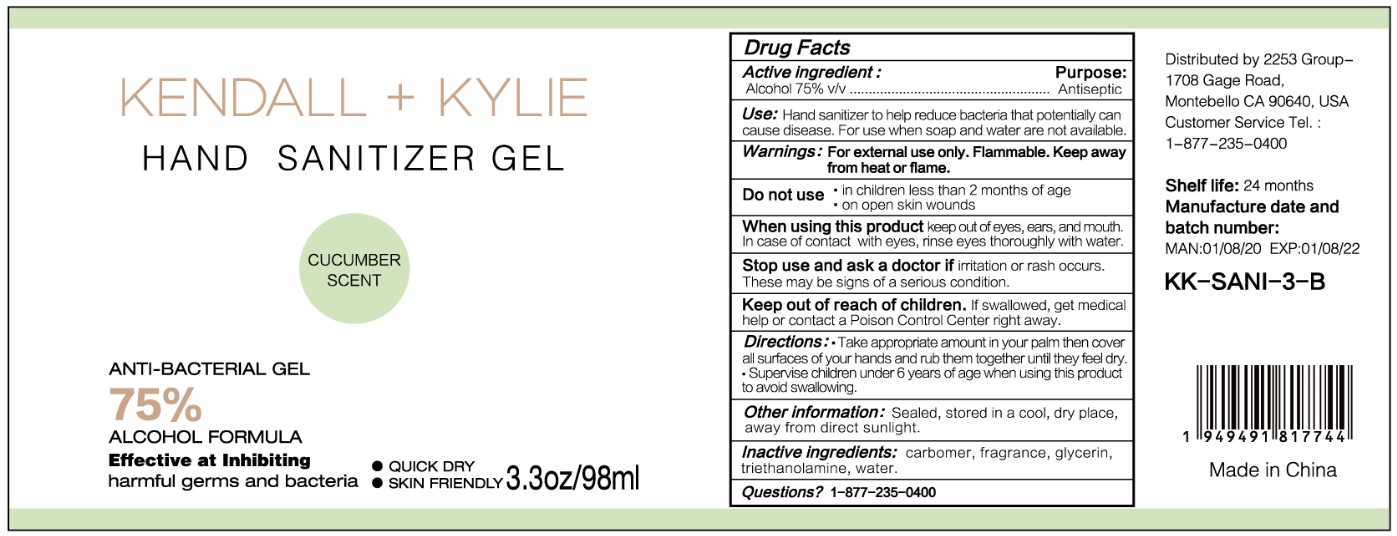
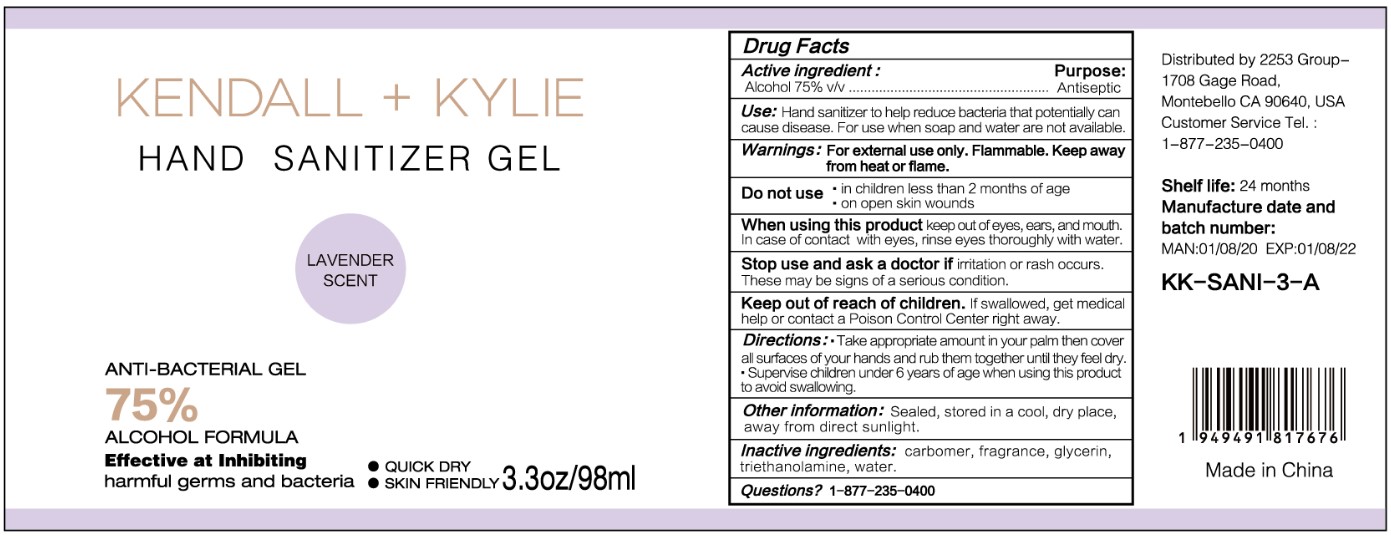
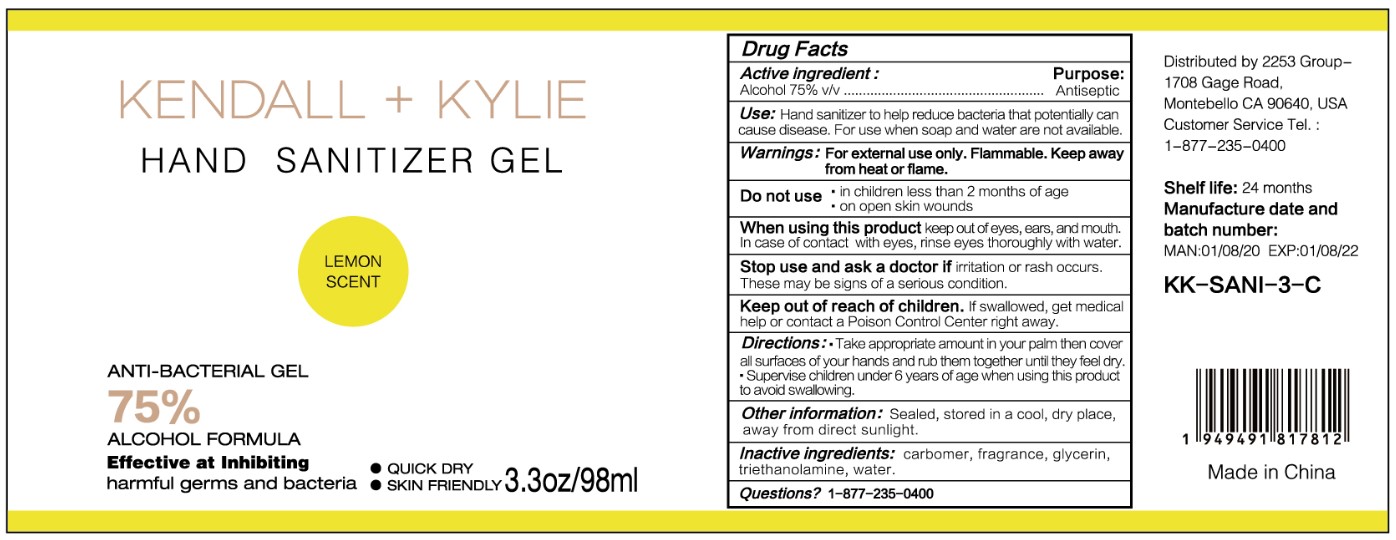
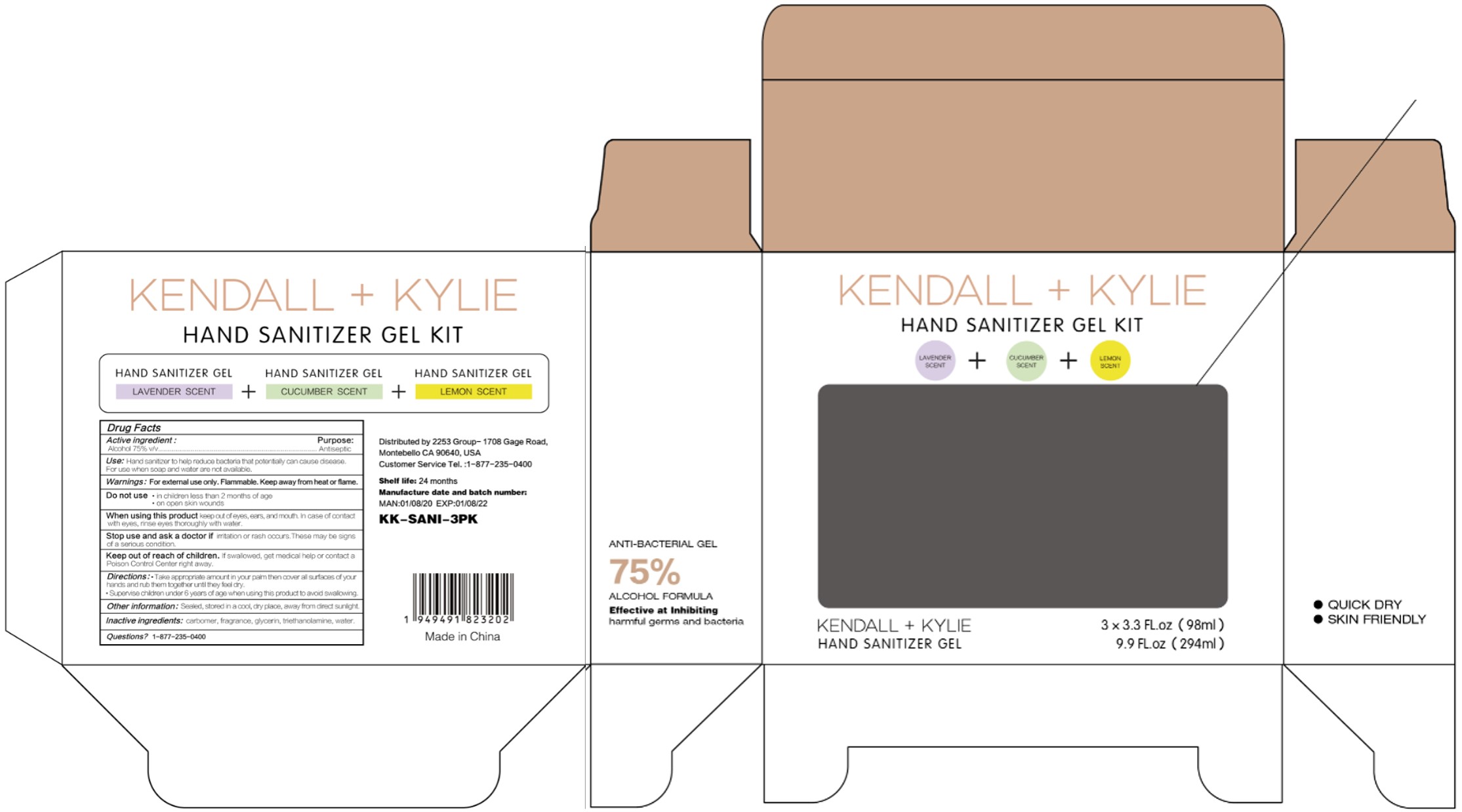
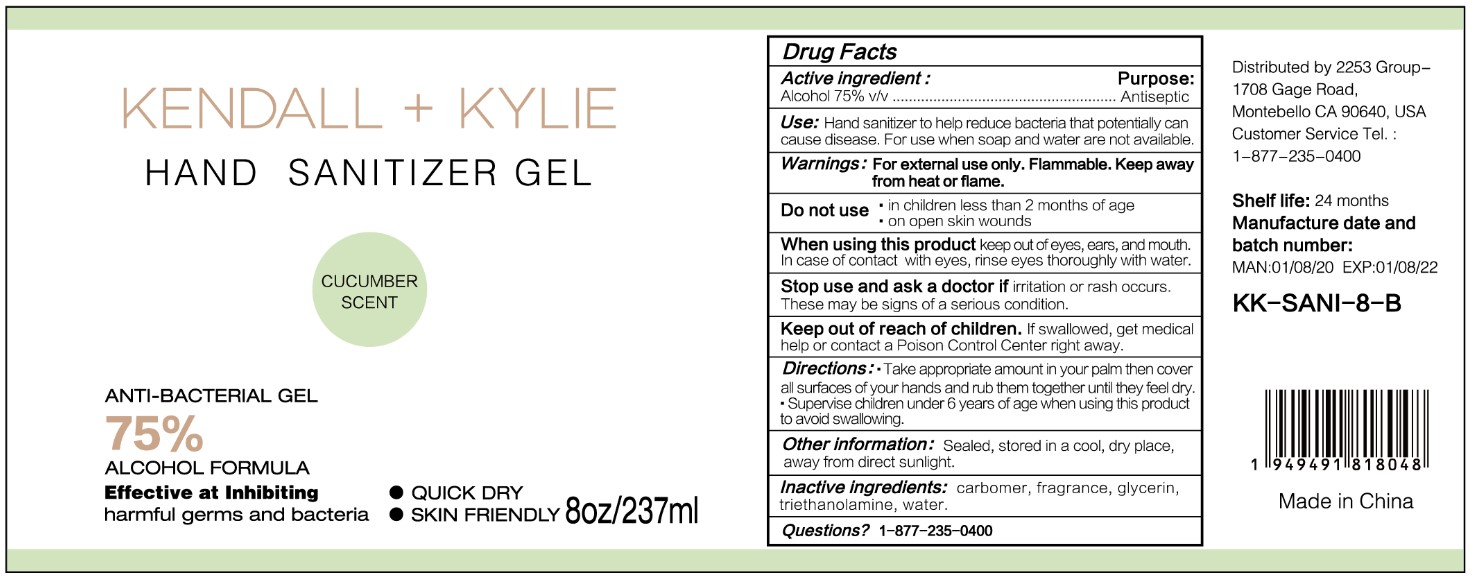
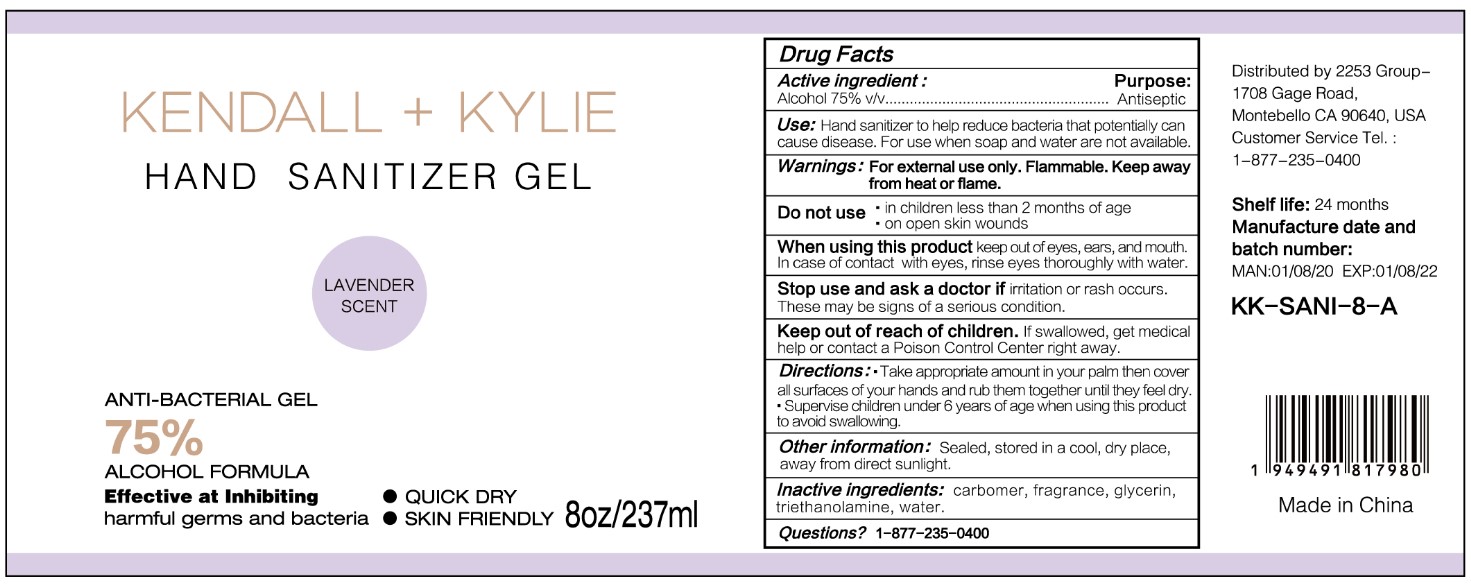
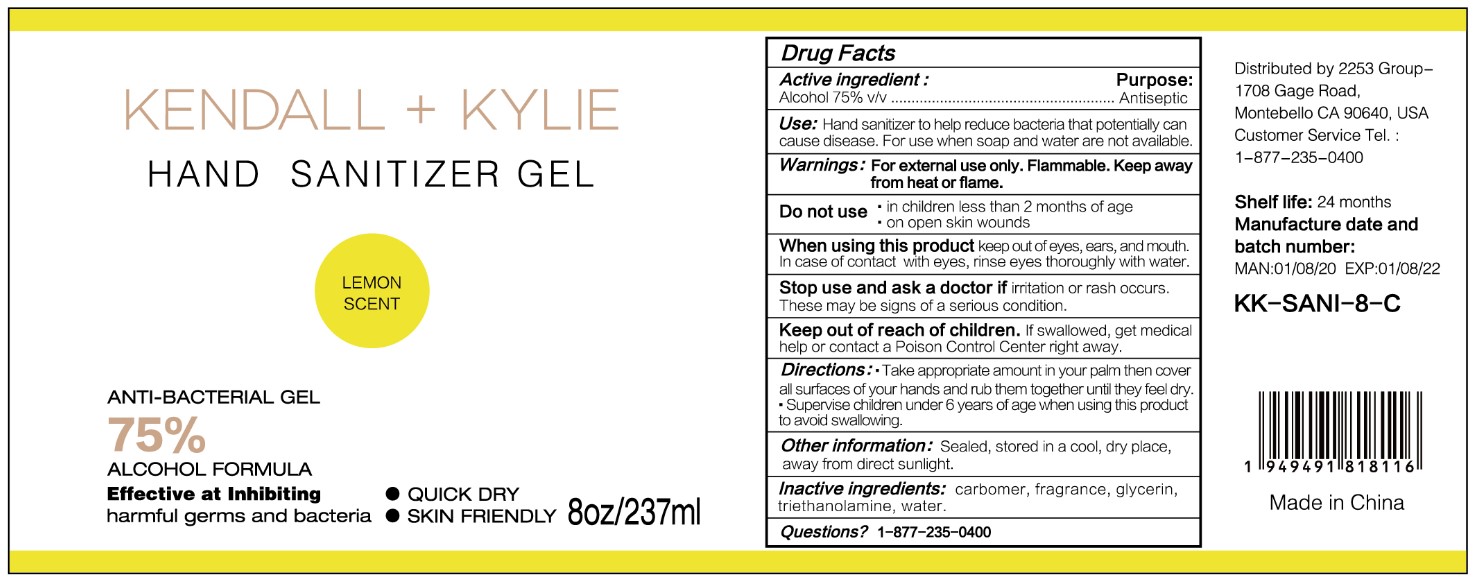
HAND SANITIZER GEL- hand sanitizer gel
Guangzhou Opseve Cosmetics Co., Ltd
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Use
Hand Sanitizer to help reduce bacteria that potentially can cause disease. For use when soap and water are not available.
When using this product keep out of eyes, ears, and mouth. In case of contact with eyes, rinse eyes thoroughly with water.
Stop use and ask a doctor if irritation or rash occurs. These may be signs of a serious condition.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
| HAND SANITIZER GEL
hand sanitizer gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Guangzhou Opseve Cosmetics Co., Ltd (421275457) |
| Registrant - Guangzhou Opseve Cosmetics Co., Ltd (421275457) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Guangzhou Opseve Cosmetics Co., Ltd | 421275457 | manufacture(77900-2022) | |