SAVIE Blue Light Hand Sanitizer
SAVIE Hand Sanitizer by
Drug Labeling and Warnings
SAVIE Hand Sanitizer by is a Otc medication manufactured, distributed, or labeled by AVB COMERCIO IMPORTACAO E DISTRIBUICAO EIRELI. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
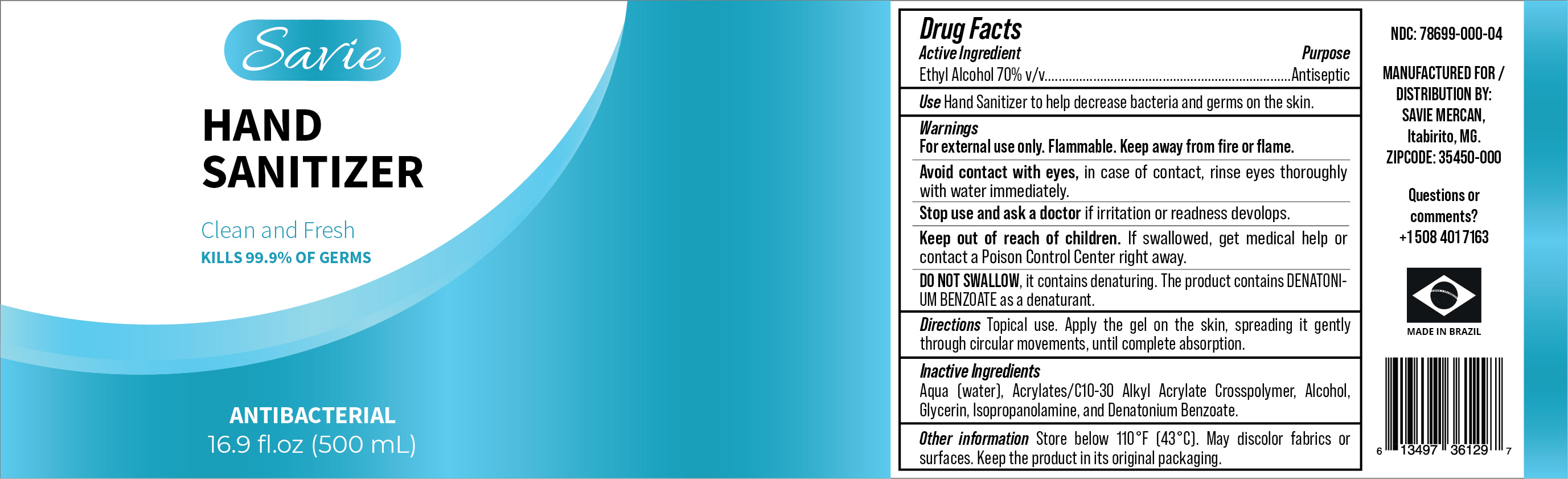
SAVIE HAND SANITIZER- alcohol gel
AVB COMERCIO IMPORTACAO E DISTRIBUICAO EIRELI
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
SAVIE Blue Light Hand Sanitizer
Warnings:
For external use only. Flammable. Keep away from fire or flame.
Directions
Topical use. Apply the gel on the skin, spreading it gently through circular movements, until complete absorption.
Other Information.
Store below 110°F (43°C). May discolor fabrics or surfaces. Keep the product in its original packaging.
| SAVIE HAND SANITIZER
alcohol gel |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - AVB COMERCIO IMPORTACAO E DISTRIBUICAO EIRELI (944841505) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.