TSC 99% Isopropyl Alcohol
TSC 99% Isopropyl Alcohol by
Drug Labeling and Warnings
TSC 99% Isopropyl Alcohol by is a Otc medication manufactured, distributed, or labeled by TS Cosmetic Labs, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
TSC 99% ISOPROPYL ALCOHOL- isopropyl alcohol liquid
TS Cosmetic Labs, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
TSC 99% Isopropyl Alcohol
This is a Rubbing Alcohol consisting of 70% Isopropyl Alcohol.
This Rubbing Alcohol is manufacture using only the following United States Pharmacopoeia (USP) grade ingredients in the preparation of the product (percentage in final product formulation):
- Isopropyl Alcohol (USP or meeting Criteria of USP) (99.9%, volume/volume (v/v))
- Purified water (0.1% v/v)
This firm does not add other active or inactive ingredients than those stated above.
Ask a doctor before use if you have
Ask a doctor before use if you have
- deep or puncture wounds, animal bites or serious burns.
When using this product
When using this product
- do not get into eyes
- do not apply over large areas of the body
- do not use longer than one week unless directed by a doctor
Keep out of reach of children
Keep out of reach of children. If case of ingestion, get medical help or contact a Poison Control Center immediately.
Directions
Directions:
- Clean affected area.
- Apply small amount of this product on the area 1-3 times daily. If bandaged, let dry first. May be covered with a sterile bandage.
Other information
Other Information
- Store at room temperature.
- Does not contain, nor is intended as a substitute for grain or ethyl alcohol.
- If taken internally, serious gastric disturbances will result.
Package Label - Principal Display Panel

NDC: 78056-099-08
32 oz | 236 mL
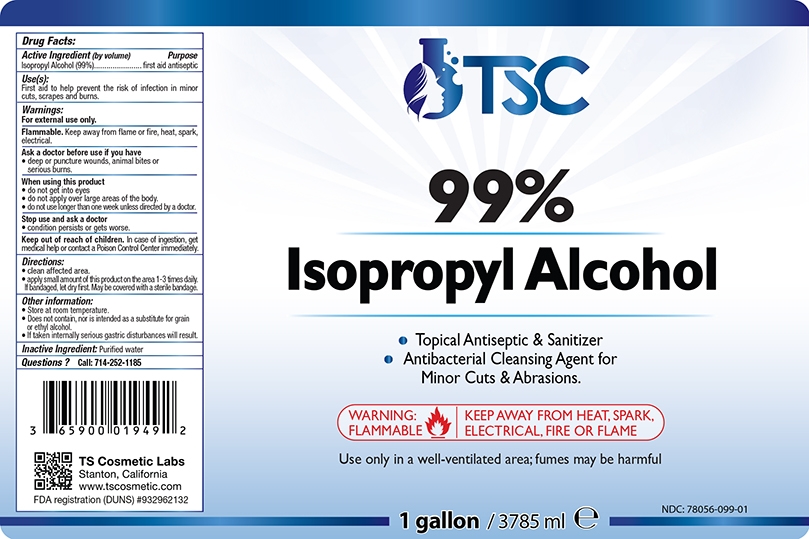
NDC: 78056-099-01
1 Gal. | 3785 mL
| TSC 99% ISOPROPYL ALCOHOL
isopropyl alcohol liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - TS Cosmetic Labs, Inc. (932962132) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.