SPOT CARE BLUE ONE by Leejiham 80395-203
SPOT CARE BLUE ONE by
Drug Labeling and Warnings
SPOT CARE BLUE ONE by is a Otc medication manufactured, distributed, or labeled by Leejiham. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
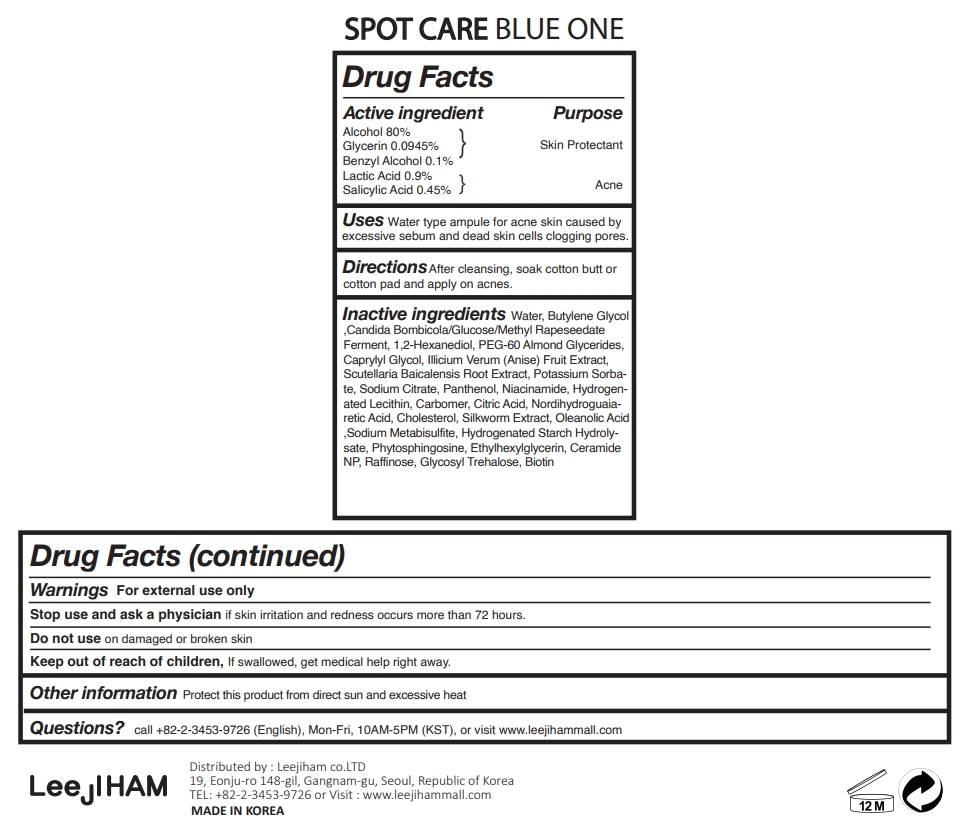
SPOT CARE BLUE ONE- alcohol, lactic acid, salicylic acid, benzyl alcohol, glycerin liquid
Leejiham
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
80395-203
Active ingredient
Alcohol 80%
Lactic Acid 0.9%
Salicylic Acid 0.45%
Glycerin 0.02%
Benzyl Alcohol 0.1%
Inactive ingredients
Alcohol, Water, Lactic Acid, Candida Bombicola/Glucose/Methyl Rapeseedate Ferment, Salicylic Acid, Phytosphingosine, Glycerin, Panthenol, Hydrogenated Lecithin, Niacinamide, Raffinose, Ceramide NP, Cholesterol, Hydrogenated Starch Hydrolysate, Glycosyl Trehalose, Ethylhexylglycerin, Silkworm Extract, Scutellaria Baicalensis Root Extract, Illicium Verum (Anise) Fruit Extract, 1,2-Hexanediol, PEG-60 Almond Glycerides, Caprylyl Glycol, Carbomer, Butylene Glycol, Nordihydroguaiaretic Acid, Oleanolic Acid, Benzyl Alcohol
| SPOT CARE BLUE ONE
alcohol, lactic acid, salicylic acid, benzyl alcohol, glycerin liquid |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Leejiham (695004132) |
| Registrant - Leejiham (695004132) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Leejiham | 695004132 | manufacture(80395-203) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.