SPOT CARE GREEN ONE by Leejiham 80395-204
SPOT CARE GREEN ONE by
Drug Labeling and Warnings
SPOT CARE GREEN ONE by is a Otc medication manufactured, distributed, or labeled by Leejiham. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SPOT CARE GREEN ONE- alcohol, benzyl alcohol liquid
Leejiham
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
80395-204
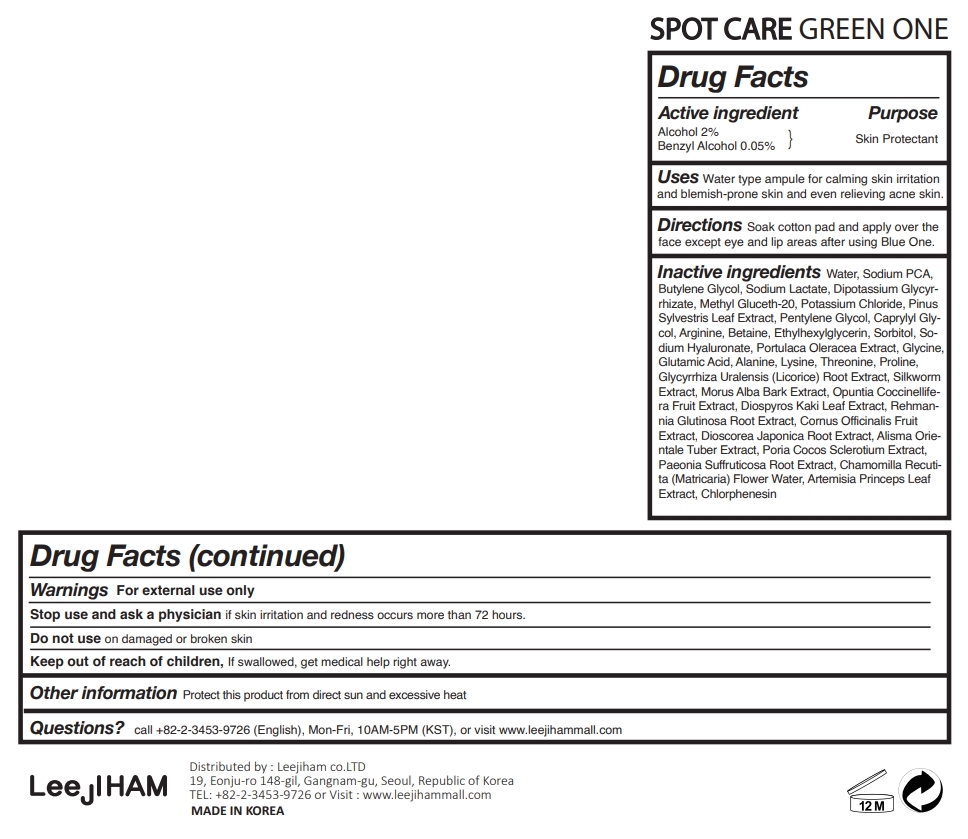
Uses
Water type ampule for calming skin irritation and blemish-prone skin and even relieving acne skin.
Inactive ingredients
Water, Alcohol, Sodium PCA, Butylene Glycol, Sodium Lactate, Dipotassium Glycyrrhizate, Methyl Gluceth-20, Potassium Chloride, Pinus Sylvestris Leaf Extract, Pentylene Glycol, Caprylyl Glycol, Arginine, Betaine, Ethylhexylglycerin, Sorbitol, Sodium Hyaluronate, Portulaca Oleracea Extract, Glycine, Glutamic Acid, Alanine, Lysine, Threonine, Proline, Glycyrrhiza Uralensis (Licorice) Root Extract, Silkworm Extract, Morus Alba Bark Extract, Opuntia Coccinellifera Fruit Extract, Diospyros Kaki Leaf Extract, Rehmannia Glutinosa Root Extract, Cornus Officinalis Fruit Extract, Dioscorea Japonica Root Extract, Alisma Orientale Tuber Extract, Poria Cocos Sclerotium Extract,Paeonia Suffruticosa Root Extract, Chamomilla Recutita (Matricaria) Flower Water, Artemisia Princeps Leaf Extract, Benzyl Alcohol, Chlorphenesin
| SPOT CARE GREEN ONE
alcohol, benzyl alcohol liquid |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Leejiham (695004132) |
| Registrant - Leejiham (695004132) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Leejiham | 695004132 | manufacture(80395-204) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.