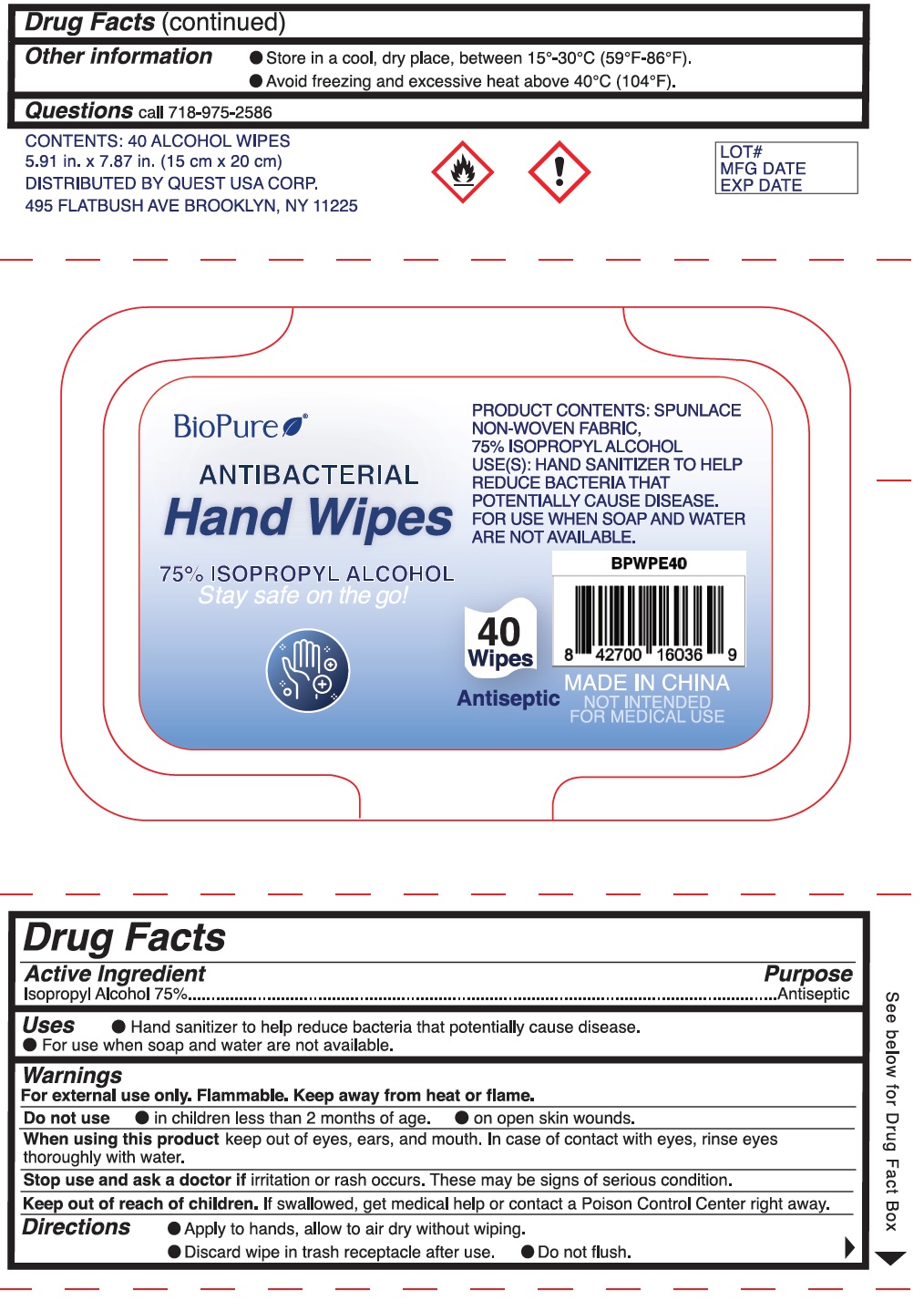
BioPure by QUEST USA CORP BioPure Wipes
BioPure by
Drug Labeling and Warnings
BioPure by is a Otc medication manufactured, distributed, or labeled by QUEST USA CORP. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
BIOPURE- isopropyl alcohol cloth
QUEST USA CORP
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
BioPure Wipes
Use
- Hand sanitizer to help reduce bacteria that potentially cause disease.
- For use when soap and water are not available.
Warnings
For external use only. Flammable, Keep away from heat or flame.
Directions
- Apply to hands, allow to air dry without wiping.
- Discard wipe in trash receptacle after use.
- Do not flush.
| BIOPURE
isopropyl alcohol cloth |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - QUEST USA CORP (079869689) |
Revised: 7/2021
Document Id: c7a56cd7-f771-437c-e053-2995a90ad27a
Set id: ae559162-05ce-3699-e053-2995a90a0480
Version: 2
Effective Time: 20210721
Trademark Results [BioPure]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BIOPURE 98218951 not registered Live/Pending |
Protein Evolution Inc. 2023-10-11 |
 BIOPURE 98199780 not registered Live/Pending |
DEUTRAMED SOLUTIONS LTD. 2023-09-27 |
 BIOPURE 98104719 not registered Live/Pending |
AGS Group, Inc. 2023-07-27 |
 BIOPURE 98011337 not registered Live/Pending |
QUEST USA CORP 2023-05-24 |
 BIOPURE 97745916 not registered Live/Pending |
Burke Texas Acquisitions, LLC 2023-01-09 |
 BIOPURE 97118461 not registered Live/Pending |
Jessica Cosmetics International, Inc. 2021-11-10 |
 BIOPURE 90367140 not registered Live/Pending |
QUEST USA CORP 2020-12-08 |
 BIOPURE 90367138 not registered Live/Pending |
QUEST USA CORP 2020-12-08 |
 BIOPURE 90367136 not registered Live/Pending |
QUEST USA CORP 2020-12-08 |
 BIOPURE 88901949 not registered Live/Pending |
QUEST USA CORP 2020-05-05 |
 BIOPURE 88878297 not registered Live/Pending |
Biopure, LLC 2020-04-19 |
 BIOPURE 88440342 not registered Live/Pending |
BioPure Services LLC 2019-05-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.