ZIIHERA- zanidatamab-hrii injection, powder, lyophilized, for solution
ZIIHERA by
Drug Labeling and Warnings
ZIIHERA by is a Prescription medication manufactured, distributed, or labeled by Jazz Pharmaceuticals, Inc., Jazz Pharmaceuticals Ireland Limited, WuXi Biologics Co., Ltd., Almac Pharma Services LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ZIIHERA safely and effectively. See full prescribing information for ZIIHERA.
ZIIHERA® (zanidatamab‑hrii) for injection, for intravenous use.
Initial U.S. Approval: 2024WARNING: EMBRYO‑FETAL TOXICITY
See full prescribing information for complete boxed warning.
- Exposure to ZIIHERA during pregnancy can cause embryo-fetal harm. Advise patients of the risk and need for effective contraception. (5.1)
INDICATIONS AND USAGE
ZIIHERA is a bispecific HER2-directed antibody indicated for the treatment of adults with previously treated, unresectable or metastatic HER2-positive (IHC 3+) biliary tract cancer (BTC), as detected by an FDA-approved test. (1)
This indication is approved under accelerated approval based on overall response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s). (1)
DOSAGE AND ADMINISTRATION
- Premedicate patients with acetaminophen, an antihistamine and a corticosteroid, 30‑60 minutes prior to each administration of ZIIHERA infusion to prevent potential infusion-related reactions (IRRs). (2.2)
- The recommended dosage of ZIIHERA is 20 mg/kg given as an intravenous infusion once every 2 weeks. (2.3)
DOSAGE FORMS AND STRENGTHS
For injection: 300 mg lyophilized powder in a single-dose vial. (3)
CONTRAINDICATIONS
- None. (4)
WARNINGS AND PRECAUTIONS
- Left Ventricular Dysfunction: Assess left ventricular ejection fraction (LVEF) prior to initiation of ZIIHERA and at regular intervals during treatment. Withhold or permanently discontinue ZIIHERA based on severity. (2.4, 5.2)
- Infusion-Related Reactions (IRRs): Premedicate before each infusion of ZIIHERA. Interrupt the infusion, decrease the infusion rate, and/or permanently discontinue ZIIHERA based on severity. (2.4, 5.3)
- Diarrhea: ZIIHERA can cause severe diarrhea. Administer antidiarrheal treatment as clinically indicated. Withhold or permanently discontinue ZIIHERA based on severity. (2.4, 5.4)
ADVERSE REACTIONS
Most common adverse reactions (≥ 20%) are diarrhea, infusion-related reaction, abdominal pain, and fatigue. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Jazz Pharmaceuticals, Inc. at 1‑800‑520‑5568 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
- Females and Males of Reproductive Potential: Verify the pregnancy status of females prior to initiation of ZIIHERA. (8.3)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 7/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: EMBRYO-FETAL TOXICITY
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection
2.2 Premedications
2.3 Recommended Dosage
2.4 Dosage Modifications for Adverse Reactions
2.5 Preparation and Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Embryo-Fetal Toxicity
5.2 Left Ventricular Dysfunction
5.3 Infusion-Related Reactions
5.4 Diarrhea
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- BOXED WARNING (What is this?)
-
1 INDICATIONS AND USAGE
ZIIHERA is indicated for the treatment of adults with previously treated, unresectable or metastatic HER2-positive (IHC 3+) biliary tract cancer (BTC), as detected by an FDA-approved test [see Dosage and Administration (2.1)].
This indication is approved under accelerated approval based on overall response rate and duration of response [see Clinical Studies (14)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s).
-
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection
Select patients for treatment of unresectable or metastatic biliary tract cancer based on HER2-positive (IHC 3+) tumor specimens, as detected by an FDA-approved test [see Clinical Studies (14)].
Information on FDA-approved tests for HER2 protein expression in biliary tract cancers is available at: http://www.fda.gov/CompanionDiagnostics.
2.2 Premedications
Premedicate all patients 30 to 60 minutes prior to each dose of ZIIHERA to reduce the risk of infusion-related reactions [see Warnings and Precautions (5.3)]:
- Administer acetaminophen, an antihistamine (such as diphenhydramine) and a corticosteroid (such as hydrocortisone).
2.3 Recommended Dosage
Recommended Dosage and Administration
The recommended dosage of ZIIHERA is 20 mg/kg, administered as an intravenous infusion once every 2 weeks until disease progression or unacceptable toxicity.
Missed dose
If a planned dose of ZIIHERA is delayed or missed, administer the dose as soon as possible; do not wait until the next planned dose. Adjust the administration schedule to maintain a 2-week interval between doses.
2.4 Dosage Modifications for Adverse Reactions
- The recommended dosage reduction of ZIIHERA for adverse reactions is 15 mg/kg as described in Table 1.
- Permanently discontinue ZIIHERA in patients who cannot tolerate 15 mg/kg.
Table 1 Dosage Modifications for Adverse Reactions Adverse Reaction Severity Treatment Modification Left Ventricular Dysfunction (LVD)
[see Warnings and Precautions (5.2)]
Absolute decrease of ≥ 16% points in LVEF from pre-treatment baseline
or
LVEF ≤ 50% and absolute decrease of ≥ 10% points below pre-treatment baseline
- Withhold ZIIHERA for at least 4 weeks.
- Repeat LVEF assessment within 4 weeks.
- Resume ZIIHERA treatment within 4 to 8 weeks if LVEF returns to normal limits and the absolute decrease is ≤ 15% points from baseline.
- Permanently discontinue ZIIHERA if LVEF has not recovered to within 15% points from pre-treatment baseline.
Confirmed symptomatic congestive heart failure
- Permanently discontinue ZIIHERA.
Infusion-Related Reactions
[see Warnings and Precautions (5.3)]
Mild (Grade 1)
- Reduce ZIIHERA infusion rate by 50%.
- For subsequent ZIIHERA infusions increase infusion rate gradually to the rate prior to the adverse reaction, as tolerated.
Moderate (Grade 2)
- Stop ZIIHERA infusion immediately.
- Treat with appropriate therapy.
- Resume ZIIHERA infusion at 50% of previous infusion rate once symptoms resolve.
- For subsequent ZIIHERA infusions increase infusion rate gradually to the rate prior to the adverse reaction, as tolerated.
Severe (Grade 3)
- Stop ZIIHERA infusion immediately.
- Promptly treat with appropriate therapy; infusion should not be restarted during the same cycle even if signs and symptoms completely resolve.
- Subsequent ZIIHERA infusions should be administered at 50% of previous infusion rate.
- Permanently discontinue ZIIHERA for recurrent Grade 3 reaction.
Life threatening (Grade 4)
- Stop ZIIHERA infusion immediately and permanently discontinue.
- Promptly treat with appropriate therapy.
Diarrhea
[see Warnings and Precautions (5.4)]
Mild/Moderate (Grade 1 or 2)
- No dose modification of ZIIHERA is required.
- Initiate appropriate medical therapy and monitor as clinically indicated.
Severe (Grade 3)
- Withhold ZIIHERA treatment until severity improves to ≤ Grade 1.
- Initiate or intensify appropriate medical therapy and monitor as clinically indicated.
- Administer subsequent ZIIHERA treatment at the same dose level or consider dose reduction to 15 mg/kg.
-
For recurrent Grade 3 symptoms, withhold ZIIHERA treatment and ensure medical management has been optimized.
- o Resume ZIIHERA treatment at a reduced dose of 15 mg/kg after severity improves to ≤ Grade 1.
- o Permanently discontinue ZIIHERA for recurrent Grade 3 symptoms that last > 3 days despite optimized medical management.
Life threatening (Grade 4)
- Permanently discontinue ZIIHERA
Pneumonitis
[see Adverse Reactions (6.1)]
Confirmed Grade ≥ 2
- Permanently discontinue ZIIHERA.
Other Adverse Reactions (excluding LVD, IRR, Diarrhea, and Pneumonitis)
[see Adverse Reactions (6.1)]
Mild/Moderate (Grades 1/2)
- No dosage modification is required for ZIIHERA.
- Initiate appropriate medical therapy and monitor as clinically indicated.
Severe (Grade 3)
- Withhold ZIIHERA treatment until severity improves to ≤ Grade 1.
- Initiate appropriate medical therapy and monitor as clinically indicated.
- Administer subsequent ZIIHERA treatment at the same dose; consider dose reduction to 15 mg/kg if Grade 3 symptoms recur.
Life Threatening (Grade 4)
- Permanently discontinue ZIIHERA, except as noted below.
- Initiate appropriate medical therapy and monitor as clinically indicated.
- ZIIHERA treatment may be resumed at the same dose level for Grade 4 electrolyte imbalances or laboratory abnormalities that are corrected within 3 days of onset; do not resume until symptoms improve to ≤ Grade 1.
- Permanently discontinue ZIIHERA for recurrent Grade 4 electrolyte imbalances or laboratory abnormalities.
2.5 Preparation and Administration Instructions
Administer only as an intravenous infusion after ZIIHERA is reconstituted and diluted.
Reconstitution
- Calculate the recommended dose based on the patient’s weight to determine the number of vials needed.
- Remove the vial(s) from the refrigerator and allow the vial(s) to reach room temperature.
- Reconstitute each 300 mg vial of ZIIHERA with 5.7 mL of Sterile Water for Injection by slowly directing the stream toward the inside of the wall of the vial, to obtain a final concentration of 50 mg/mL in an extractable volume of 6 mL.
- Swirl the vial gently until completely dissolved. Do not shake or vigorously swirl.
- Allow the reconstituted vial to settle to allow bubbles to dissipate.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. The reconstituted product should be a colorless to light yellow, clear to slightly opalescent solution with no visible particles. Discard the reconstituted vial if any discoloration or particulate matter is observed.
- The product does not contain a preservative. Use the reconstituted ZIIHERA solution immediately or store the reconstituted ZIIHERA solution for up to 4 hours, either at room temperature (18°C to 24°C [64°F to 75°F]) or in a refrigerator (2°C to 8ºC [36°F to 46ºF]).
Dilution
- Withdraw the necessary volume for the calculated dose from each vial [see Dosage and Administration (2.3)].
- Slowly add the necessary dose volume to an infusion bag containing 0.9% Sodium Chloride Injection or 5% Dextrose Injection to prepare an infusion solution with a final concentration of the diluted solution between 0.4 mg/mL and 6 mg/mL.
- Gently invert the infusion bag to mix. Do not shake.
- The infusion solution must be a clear, colorless solution with no visible particles. Do not use if visible particles are observed or if the solution is discolored.
- Discard any unused portion left in the vial(s).
-
Use the infusion solution immediately upon dilution or store the infusion solution at room temperature (18°C to 24°C [64°F to 75°F]) for up to 12 hours or in the refrigerator (2ºC to 8ºC [36ºF to 46ºF]) for up to 24 hours.
- o These time limits include the beginning of reconstitution through the duration of infusion.
- o If these specified times are exceeded, discontinue the current infusion bag and prepare a new bag which contains the remaining dosage of ZIIHERA to be infused.
-
Compatibility with intravenous administration materials and the infusion solution has been demonstrated in the following materials:
- o Intravenous (IV) Bag: Polyvinyl chloride (PVC), polyolefin (PO), ethyl vinyl acetate (EVA), polypropylene (PP) and ethylene-propylene copolymer.
- o Infusion sets: Polyvinyl chloride/ bis (2-ethylhexyl) phthalate (PVC/DEHP). Polyurethane (PUR), polyethylene-lined (PE-lined) acrylonitrile-butadiene-styrene (ABS).
- o Inline filters: Polyethersulfone solution filter (PES), polyvinylidene fluoride air filter (PVDF).
- o Closed System Transfer devices: acrylonitrile-butadiene-styrene (ABS), acrylic c-polymer, polycarbonate (PC), polyisoprene (PI), polyester, polypropylene (PP) polytetrafluoroethylene (PTFE), silicone and stainless steel (SS).
Administration
- Administer ZIIHERA as an intravenous infusion with a 0.2 or 0.22 micron filter.
- Do not administer as an intravenous push or bolus.
- Do not co-administer ZIIHERA and other intravenous drugs through the same intravenous line.
Table 2: Recommended ZIIHERA Infusion Durations Dose
Infusion Duration
First and Second
120-150 minutes
Third and Fourth
90 minutes, if previous infusions were well-tolerated
Subsequent
60 minutes, if previous infusions were well-tolerated
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Embryo-Fetal Toxicity
Based on the mechanism of action, ZIIHERA can cause fetal harm when administered to a pregnant woman. There are no human or animal data on the use of ZIIHERA in pregnancy. In literature reports, use of a HER2-directed antibody during pregnancy resulted in cases of oligohydramnios and oligohydramnios sequence manifesting as pulmonary hypoplasia, skeletal abnormalities, and neonatal death.
Verify the pregnancy status of females of reproductive potential prior to the initiation of ZIIHERA. Advise pregnant women and females of reproductive potential that exposure to ZIIHERA during pregnancy or within 4 months prior to conception can result in fetal harm. Advise females of reproductive potential to use effective contraception while receiving ZIIHERA and for 4 months following the last dose of ZIIHERA.
5.2 Left Ventricular Dysfunction
ZIIHERA can cause decreases in left ventricular ejection fraction (LVEF). LVEF declined by greater than 10% and decreased to less than 50% in 4.3% of 233 patients. LVD leading to permanent discontinuation of ZIIHERA was reported in 0.9% of patients. The median time to first occurrence of left ventricular dysfunction was 5.6 months (range: 1.6 to 18.7 months). Left ventricular dysfunction resolved in 70% of patients.
Assess LVEF prior to initiation of ZIIHERA and at regular intervals during treatment. Withhold dose or permanently discontinue ZIIHERA based on severity of adverse reactions [see Dosage and Administration (2.4)].
The safety of ZIIHERA has not been established in patients with a baseline ejection fraction that is below 50% [see Dosage and Administration (2.4)].
5.3 Infusion-Related Reactions
ZIIHERA can cause infusion-related reactions (IRRs). An IRR was reported in 31% of 233 patients treated with ZIIHERA as a single agent in clinical studies, including Grade 3 (0.4%), and Grade 2 (25%). IRRs leading to permanent discontinuation of ZIIHERA were reported in 0.4% of patients. IRRs occurred on the first day of dosing in 28% of patients; 97% of IRRs resolved within one day.
Prior to each dose of ZIIHERA, administer premedications to prevent potential IRRs [see Dosage and Administration (2.2)]. Monitor patients for signs and symptoms of IRR during ZIIHERA administration and as clinically indicated after completion of infusion. Have medications and emergency equipment to treat IRRs available for immediate use.
If an IRR occurs, slow, or stop the infusion, and administer appropriate medical management. Monitor patients until complete resolution of signs and symptoms before resuming. Permanently discontinue ZIIHERA in patients with recurrent severe or life-threatening infusion-related reactions [see Dosage and Administration (2.4)].
5.4 Diarrhea
ZIIHERA can cause severe diarrhea [see Adverse Reactions (6.1)].
Diarrhea was reported in 48% of 233 patients treated in clinical studies, including Grade 3 (6%) and Grade 2 (17%). If diarrhea occurs, administer antidiarrheal treatment as clinically indicated. Perform diagnostic tests as clinically indicated to exclude other causes of diarrhea. Withhold or permanently discontinue ZIIHERA based on severity [see Dosage and Administration (2.4)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described in greater detail in other sections of the labeling:
- Embyro-Fetal Toxicity [see Warnings and Precautions (5.1)]
- Left Ventricular dysfunction [see Warnings and Precautions (5.2)]
- Infusion-Related Reactions [see Warnings and Precautions (5.3)]
- Diarrhea [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The pooled safety population of ZIIHERA described in WARNINGS AND PRECAUTIONS reflect exposure in 233 patients administered ZIIHERA 20 mg/kg intravenously as a single agent in two single-arm, open-label studies (ZWI‑ZW25‑101 and HERIZON‑BTC‑01), which enrolled 109 patients with biliary tract cancer, and 124 patients with other cancers. Among 233 patients who received ZIIHERA, 39% were exposed for 6 months or longer, and 17% were exposed for greater than one year.
Biliary Tract Cancer
The safety of ZIIHERA was evaluated in 80 patients with previously treated, unresectable or metastatic HER2-positive biliary tract cancer who received at least one prior gemcitabine-containing chemotherapy regimen in HERIZON‑BTC‑01 [See Clinical Studies (14)]. Patients received ZIIHERA 20 mg/kg by IV infusion once every 2 weeks until disease progression or unacceptable toxicity. The median duration of exposure to ZIIHERA was 5.6 months (range: 0.5 to 27.2 months).
Serious adverse reactions occurred in 53% of patients who received ZIIHERA. Serious adverse reactions in > 2% of patients included biliary obstruction (15%), biliary tract infection (8%), sepsis (8%), pneumonia (5%), diarrhea (3.8%), gastric obstruction (3.8%), and fatigue (2.5%). A fatal adverse reaction of hepatic failure occurred in one patient who received ZIIHERA.
Permanent discontinuation due to an adverse reaction occurred in 2.5% of patients who received ZIIHERA. Adverse reactions which resulted in permanent discontinuation in ≥ 1% of patients who received ZIIHERA included decreased ejection fraction and pneumonitis.
Dosage interruptions due to an adverse reaction, excluding temporary interruptions of ZIIHERA infusions due to infusion-related reactions, occurred in 41% of patients who received ZIIHERA. The most frequent adverse reactions (> 2% of patients) that required dosage interruption were diarrhea, increased alanine aminotransferase, increased aspartate aminotransferase, decreased ejection fraction, pneumonia, cholangitis, fatigue, biliary obstruction, abdominal pain, increased blood creatinine, and decreased potassium.
Dosage reductions due to an adverse reaction occurred in 4% of patients who received ZIIHERA. Adverse reactions requiring dosage reductions in > 1% of patients were diarrhea, nausea, and decreased weight.
The most common adverse reactions in patients receiving ZIIHERA (≥ 20%) were diarrhea, infusion-related reaction, abdominal pain, and fatigue.
Table 3 summarizes the adverse reactions that occurred in HERIZON‑BTC‑01.
Table 3: Adverse Reactions (≥ 15%) in Patients with Unresectable or Metastatic HER2-Positive BTC Receiving ZIIHERA in HERIZON-BTC-01 Adverse Reaction*
ZIIHERA
N=80All Grades
(%)Grades 3 or 4
(%)Gastrointestinal disorders
Diarrheaa
50
10
Abdominal painb
29
1
Nausea
18
1
Vomiting
15
1
Injury, poisoning and procedural complications
Infusion-related reaction
35
1
General disorders and administration site conditions
Fatiguec
24
4
Skin and subcutaneous tissue disorders
Rashd
19
0
Metabolism and nutrition disorders
Decreased appetite
16
0
* Graded per CTCAE version 5.
a Diarrhea includes diarrhea and enteritis
b Abdominal pain includes abdominal pain and abdominal pain upper
c Fatigue includes asthenia and fatigue
d Rash includes dermatitis, dermatitis acneiform, palmar-plantar erythrodysaesthesia syndrome, rash, rash maculo-papular, and rash pustular
Table 4 summarizes the laboratory abnormalities in HERIZON‑BTC‑01.
Table 4: Laboratory Abnormalities (≥ 30%) that Worsened from Baseline in Patients with Unresectable or Metastatic HER2-Positive BTC Receiving ZIIHERA in HERIZON-BTC-01 Laboratory Abnormalities
ZIIHERA*
All Grades
(%)Grades 3 or 4
(%)Hematology
- Hemoglobin decreased
88
14
- Lymphocytes decreased
44
8
Chemistry
- Lactate dehydrogenase increased
55
0
- Albumin decreased
53
0
- Aspartate aminotransferase increased
47
10
- Alanine aminotransferase increased
46
8
- Alkaline phosphatase increased
41
5
- Sodium decreased
35
10
- Potassium decreased
34
5
*The denominator used to calculate the rate varied from 78 to 80 based on the number of patients with a baseline value and at least one post-treatment value.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on mechanism of action, ZIIHERA can cause fetal harm when administered to a pregnant woman. There are no human or animal data on the use of ZIIHERA in pregnancy. In literature reports, use of a HER2-directed antibody during pregnancy resulted in cases of oligohydramnios and oligohydramnios sequence manifesting as pulmonary hypoplasia, skeletal abnormalities, and neonatal death. Use of ZIIHERA is not recommended during pregnancy (see CLINICAL CONSIDERATIONS). Advise patients of potential risks to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, background risks of major birth defects and miscarriage in clinically recognized pregnancies are 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Monitor women who received ZIIHERA during pregnancy or within 4 months prior to conception for oligohydramnios. If oligohydramnios occurs, perform fetal testing that is appropriate for gestational age and consistent with local standard of care.
8.2 Lactation
Risk Summary
There are no data on the presence of zanidatamab‑hrii in human milk, the effects on the breastfed child, or the effects on milk production. Published data suggest human IgG is present in human milk but does not enter neonatal or infant circulation in substantial amounts. Consider developmental and health benefits of breastfeeding along with the mother’s clinical need for ZIIHERA treatment and any potential adverse effects on the breastfed child from ZIIHERA or from the underlying maternal condition. This consideration should also take into account the ZIIHERA half-life of approximately 21 days and a washout period of 4 months [see Clinical Pharmacology (12.3)].
8.3 Females and Males of Reproductive Potential
ZIIHERA can cause fetal harm when administered to a pregnant woman [see Warnings and Precautions (5.1), Use in Specific Populations (8.1)].
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to the initiation of ZIIHERA [see Use in Specific Populations (8.1)].
Contraception
Females
ZIIHERA can cause embryo-fetal harm when administered during pregnancy. Advise females of reproductive potential to use effective contraception during treatment with ZIIHERA and for 4 months following the last dose of ZIIHERA.
8.5 Geriatric Use
Of the 80 patients who received ZIIHERA for unresectable or metastatic biliary tract cancer in HERIZON‑BTC‑01, there were 39 (49%) patients 65 years of age and older. Thirty‑seven (46%) were aged 65‑74 years old and 2 (3%) were aged 75 years or older [see Clinical Studies (14)].
No overall differences in safety or effectiveness were observed between these patients and younger adult patients.
-
11 DESCRIPTION
Zanidatamab‑hrii is a humanized, IgG-like, bispecific HER2-directed antibody. Zanidatamab‑hrii is produced in Chinese hamster ovary cells via recombinant DNA technology and has a molecular weight of 124.8 kDa.
ZIIHERA (zanidatamab‑hrii) for injection is supplied as a sterile, preservative free, white lyophilized powder that requires reconstitution and dilution for intravenous use. Each single-dose vial of reconstituted product contains 300 mg of zanidatamab‑hrii and the inactive ingredients: polysorbate 20 (0.63 mg), sodium succinate (4.3 mg), succinic acid (4.3 mg), and sucrose (567 mg). Following reconstitution with 5.7 mL Sterile Water for Injection, a solution containing 50 mg/mL zanidatamab‑hrii is produced with a deliverable volume of 6 mL, with pH of 4.6. The resulting solution is diluted and administered by intravenous infusion.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Zanidatamab‑hrii is a bispecific HER2-directed antibody that binds to two extracellular sites on HER2. Binding of zanidatamab‑hrii with HER2 results in internalization leading to a reduction of the receptor on the tumor cell surface. Zanidatamab‑hrii induces complement-dependent cytotoxicity (CDC), antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP). These mechanisms result in tumor growth inhibition and cell death in vitro and in vivo.
12.2 Pharmacodynamics
Zanidatamab‑hrii exposure-response relationships and the time course of the pharmacodynamic response are unknown.
Cardiac Electrophysiology
A mean increase in the QTc interval > 20 ms was not observed at the recommended approved dosage.
12.3 Pharmacokinetics
Zanidatamab‑hrii PK parameters are presented as means (percent coefficient of variation) following administration of ZIIHERA 20 mg/kg every 2 weeks after the 7th or later dose unless otherwise indicated.
Zanidatamab‑hrii maximum concentration (Cmax) is 600 (22.2) µg/mL, the lowest measured concentration (Ctrough) is 178 (29.6) µg/mL, and total systemic exposure (AUC0-336h) is 3,976 (22.5) days*µg/mL following administration of ZIIHERA. The Cmax of zanidatamab‑hrii is dose proportional and the total systemic exposure (AUC0-inf) of zanidatamab‑hrii is greater than dose proportional with increasing doses. The mean Ctrough accumulation ratio of zanidatamab‑hrii is approximately 2.4.
Distribution
The volume of distribution of zanidatamab‑hrii is approximately 7.5 (33) L based on the population pharmacokinetic analysis.
Elimination
The estimated half-life (t1/2) of zanidatamab‑hrii is approximately 21 days with an associated clearance (CL) of 0.012 L/h (27.9) based on the population pharmacokinetic analysis.
Metabolism
Zanidatamab‑hrii is expected to be metabolized into small peptides by catabolic pathways.
Specific Populations
No clinically significant differences in the pharmacokinetics of zanidatamab-hrii were observed based on age (24 to 88 years), sex, race (White and Asian), mild and moderate renal impairment (eGFR 30 to 89 mL/min estimated using the CKD‑EPI), mild hepatic impairment (total bilirubin ≤ upper limit of normal (ULN) and AST > ULN or total bilirubin between 1 and 1.5 times ULN and any AST), or body weight (35 kg to 128 kg).
The effect of severe renal impairment (eGFR 15 to 29 mL/min), end-stage renal disease (eGFR < 15 mL/min) with or without hemodialysis, and moderate (total bilirubin > 1.5 to ≤ 3 ULN and any AST) or severe (total bilirubin > 3 ULN and any AST) hepatic impairment on the pharmacokinetics of zanidatamab‑hrii is unknown.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
HER2-positive (IHC 3+) Biliary Tract Cancer (BTC)
The efficacy of ZIIHERA was evaluated in 62 patients with HER2-positive (IHC 3+ by central assessment) BTC in Cohort 1 of HERIZON‑BTC‑01 (NCT04466891), an open-label, multicenter, single arm trial in patients with unresectable or metastatic disease. Patients were required to have received at least one prior gemcitabine-containing systemic chemotherapy regimen in the advanced disease setting and adequate cardiac function (defined as LVEF ≥ 50%).
Patients received ZIIHERA 20 mg/kg intravenously every 2 weeks. ZIIHERA was administered until disease progression or unacceptable toxicity. The major efficacy outcome measures were objective response rate (ORR) and duration of response (DOR) as determined by an independent central review (ICR) according to Response Evaluation Criteria in Solid Tumors (RECIST) v1.1.
The median age was 64 years (range: 38 to 79 years), 47% of patients were age 65 or older; 55% were female; 61% were Asian, 31% were White, 2% were American Indian or Alaskan Native and for 6% race was unknown or not reported; 89% were Non-Hispanic or Latino, 8% Hispanic/Latino, and for 3% ethnicity was unknown or not reported. All patients had a baseline Eastern Cooperative Oncology Group (ECOG) performance status of 0 (32%) or 1 (68%). Fifty-three percent of patients had gallbladder cancer, 27% had intrahepatic cholangiocarcinoma, and 19% had extrahepatic cholangiocarcinoma. All patients received at least 1 prior line of gemcitabine-based therapy, 31% had 2 prior lines of therapy, and 10% had 3 or more prior lines of therapy for unresectable or metastatic disease.
Efficacy results are summarized in Table 5.
Table 5 Efficacy Results in HERIZON-BTC-01 Efficacy Parameter*
ZIIHERA
(N=62)Objective Response Rate (95% CI)
52% (39, 65)
- Complete response, n (%)
2 (3.2)
- Partial response, n (%)
30 (48)
Duration of Response (DOR)
N=32
- Median†, months (95% CI)
14.9 (7.4, NE)
- DOR ≥ 6 months, n (%) ‡
(19 59)
- DOR ≥ 12 months, n (%) ‡
14 (44)
*Assessed by independent central review
† Based on Kaplan-Meier estimate
‡ Based on observed duration of response
NE = not estimable
-
16 HOW SUPPLIED/STORAGE AND HANDLING
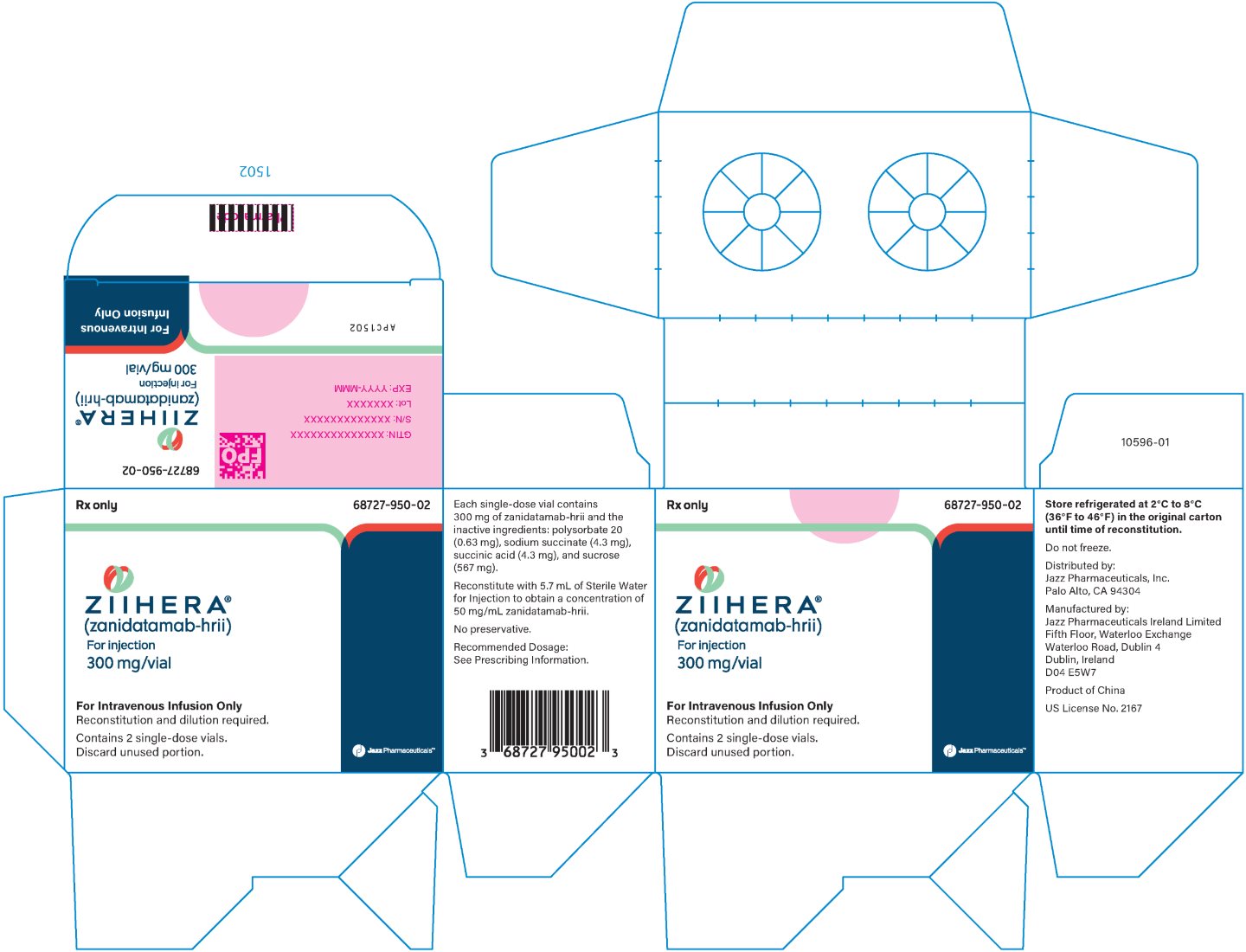
ZIIHERA is supplied as a sterile, preservative free, white lyophilized powder in a single-dose vial. Each single-dose vial (NDC 68727‑950‑01) contains 300 mg zanidatamab‑hrii. Each carton of ZIIHERA (NDC 68727‑950‑02) contains 2 single-dose vials.
Store in a refrigerator at 2ºC to 8ºC (36ºF to 46ºF) in the original carton until time of reconstitution. Do not freeze.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Embryo-Fetal Toxicity
Advise female patients of the potential risk to a fetus. Advise female patients to contact their healthcare provider with a known or suspected pregnancy [see Warnings and Precautions (5.1), Use in Specific Populations (8.1)].
Advise females of reproductive potential to use effective contraception during treatment with ZIIHERA and for 4 months following the last dose [see Warnings and Precautions (5.1), Use in Specific Populations (8.3)].
Left Ventricular Dysfunction
Advise patients that ZIIHERA can cause cardiac dysfunction and to contact a healthcare provider immediately for signs and symptoms of cardiac dysfunction [see Warnings and Precautions (5.2)].
Infusion-Related Reactions
Advise patients of the risk of infusion-related reactions and to inform a healthcare provider immediately for symptoms of an infusion-related reaction [see Warnings and Precautions (5.3)].
Diarrhea
Advise patients that ZIIHERA can cause diarrhea, which may be severe. Instruct patients how to manage diarrhea, and to contact their healthcare provider for sustained diarrhea that does not respond to supportive care [see Dosage and Administration (2.4), Warnings and Precautions (5.4)].
Distributed by:
Jazz Pharmaceuticals, Inc.
Palo Alto, CA 94306Manufactured by:
Jazz Pharmaceuticals Ireland Limited
Fifth Floor, Waterloo Exchange
Waterloo Road, Dublin 4
Dublin, Ireland
D04 E5W7U.S. License No. 2167
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
ZIIHERA (zye HAYR rah)
(zanidatamab-hrii)
for injection, for intravenous useWhat is the most important information I should know about ZIIHERA?
ZIIHERA can cause serious side effects, including:
-
harm to your unborn baby. Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with ZIIHERA.
- o If you are able to become pregnant, your healthcare provider should do a pregnancy test before you start treatment with ZIIHERA.
- o Females who are able to become pregnant should use birth control (contraception) during treatment with ZIIHERA and for 4 months after the last dose.
See “What are the possible side effects of ZIIHERA?” for more information about side effects.
What is ZIIHERA?
ZIIHERA is a prescription medicine used to treat adults who have a type of bile duct (cholangiocarcinoma) or gallbladder cancer called biliary tract cancer (BTC), that is human epidermal growth factor receptor 2 (HER2)-positive (IHC 3+). ZIIHERA may be used when your BTC:
-
- o was previously treated;
- o cannot be removed by surgery or has spread to other parts of your body (metastatic).
Your healthcare provider will perform a test to make sure ZIIHERA is right for you.
It is not known if ZIIHERA is safe and effective in children.
Before receiving ZIIHERA, tell your healthcare provider about all of your medical conditions, including if you:
- have or have had any heart problems
- are breastfeeding or plan to breastfeed. It is not known if ZIIHERA passes into your breastmilk. Talk to your healthcare provider about the best way to feed your baby if you receive treatment with ZIIHERA.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I receive ZIIHERA?
- ZIIHERA is given through a vein by an intravenous (IV) infusion over 120 to 150 minutes for your first and second infusions. If you tolerate the first and second infusions well, your third and fourth infusions may be given to you over 90 minutes. Each additional infusion may be given to you over 60 minutes.
- ZIIHERA is given every 2 weeks.
-
Your healthcare provider:
- o will give you medicines 30 to 60 minutes before each treatment to help prevent infusion-related reactions or make them less severe
- o may slow down, temporarily stop, or permanently stop your treatment with ZIIHERA if you have certain side effects
- o may do certain tests to check you for some side effects
- o will decide how many treatments you need
- If you miss a dose, call your healthcare provider as soon as possible to reschedule your appointment.
What are the possible side effects of ZIIHERA?
ZIIHERA can cause serious side effects, including:
- See “What is the most important information I should know about ZIIHERA?”
-
Heart problems that may affect how well your heart pumps blood. Your healthcare provider will check your heart function before and during treatment with ZIIHERA. Call your healthcare provider right away if you get any of the following signs and symptoms of a heart problem:
- o new or worsening shortness of breath
- o feeling more tired than usual
- o swelling of your ankles or feet
- o loss of consciousness
- o irregular heartbeat
- o sudden weight gain
- o dizziness or feeling light-headed
-
Infusion-related reactions, a common side effect that can happen during treatment with ZIIHERA. Your healthcare provider will check for side effects during your infusions. Tell your healthcare provider right away if you get any of the following symptoms during or after your infusions of ZIIHERA:
- o shortness of breath or trouble breathing
- o fever
- o chills
- o rash
- o flushing
- o nausea or vomiting
- o dizziness or feeling lightheaded
- o chest discomfort
- Diarrhea, a common side effect during treatment with ZIIHERA that may be severe. Your healthcare provider may prescribe an antidiarrheal medicine or other treatment, as needed.
Other common side effects of ZIIHERA include stomach pain and feeling tired.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all of the possible side effects of ZIIHERA.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1‑800‑FDA‑1088.
General information about the safe and effective use of ZIIHERA.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your pharmacist or healthcare provider for information about ZIIHERA that is written for health professionals.
What are the ingredients in ZIIHERA?
Active ingredient: zanidatamab-hrii
Inactive ingredients: polysorbate 20, sodium succinate, succinic acid, and sucrose
Distributed by:
Jazz Pharmaceuticals, Inc.
Palo Alto, CA 94306
Manufactured by:
Jazz Pharmaceuticals Ireland Limited
Fifth Floor, Waterloo Exchange
Waterloo Road, Dublin 4
Dublin, Ireland
D04 E5W7
U.S. License No. 2167
For more information, go to www.ZIIHERA.com or call 1‑800‑520‑5568.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: 7/2025
-
harm to your unborn baby. Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with ZIIHERA.
- Package/Label Display Panel
- Package/Label Display Panel
-
INGREDIENTS AND APPEARANCE
ZIIHERA
zanidatamab-hrii injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68727-950 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZANIDATAMAB (UNII: Z20OC92TDI) (ZANIDATAMAB - UNII:Z20OC92TDI) ZANIDATAMAB 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM SUCCINATE ANHYDROUS (UNII: V8ZGC8ISR3) 4.3 mg in 1 mL POLYSORBATE 20 (UNII: 7T1F30V5YH) 0.63 mg in 1 mL SUCCINIC ACID (UNII: AB6MNQ6J6L) 4.3 mg in 1 mL SUCROSE (UNII: C151H8M554) 567 mg in 1 mL Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68727-950-02 2 in 1 CARTON 11/20/2024 1 NDC: 68727-950-01 6 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761416 11/20/2024 Labeler - Jazz Pharmaceuticals, Inc. (135926363) Registrant - Jazz Pharmaceuticals Ireland Limited (896650210) Establishment Name Address ID/FEI Business Operations WuXi Biologics Co., Ltd. 421298354 MANUFACTURE(68727-950) , ANALYSIS(68727-950) Establishment Name Address ID/FEI Business Operations Almac Pharma Services LLC 078607239 PACK(68727-950) , LABEL(68727-950) Establishment Name Address ID/FEI Business Operations Jazz Pharmaceuticals Ireland Limited 896650210 MANUFACTURE(68727-950)
Trademark Results [ZIIHERA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ZIIHERA 79344656 not registered Live/Pending |
Zymeworks Inc. 2022-06-09 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.