Safecare Premium Sanitizing Alcohol Wipes
Safecare Premium Sanitizing Alcohol Wipes by
Drug Labeling and Warnings
Safecare Premium Sanitizing Alcohol Wipes by is a Otc medication manufactured, distributed, or labeled by Xiamen Yiukin Paper Products Co.,ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SAFECARE PREMIUM SANITIZING ALCOHOL WIPES- alcohol cloth
Xiamen Yiukin Paper Products Co.,ltd
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Safecare Premium Sanitizing Alcohol Wipes
Uses
- For hand washng to decrease bacteria that potentially can cause disease.
- Antiseptic for hands and skin.
- Sanitize hands and skin when water and soap are not available.
Warnings
- For external use only. DO NOT INGEST.
Warning: Flammable. Do not use near open flame, fire or spark.
Do not use
On infants under 2 months old.
On open skin or puncture-type wounds.
Near or in eyes. If eye contact occurs, flush with cold water.
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Directions for use
- Clean hands and skin with wipe by rubbing with wipe.
- No need to rinse, let hands or skin dry.
Other information
- Read all directions and warnings before using hand wipes.
- Store at room temperature 15 to 30 C (59 to 86 F) with package lid closed.
- Avoid excess heat above 40 C (104 F)
Inactive ingredients
Water, Benzalkonium Chloride, Cetylpyridinium chloride, Phenoxyethanol, Propylene glycol
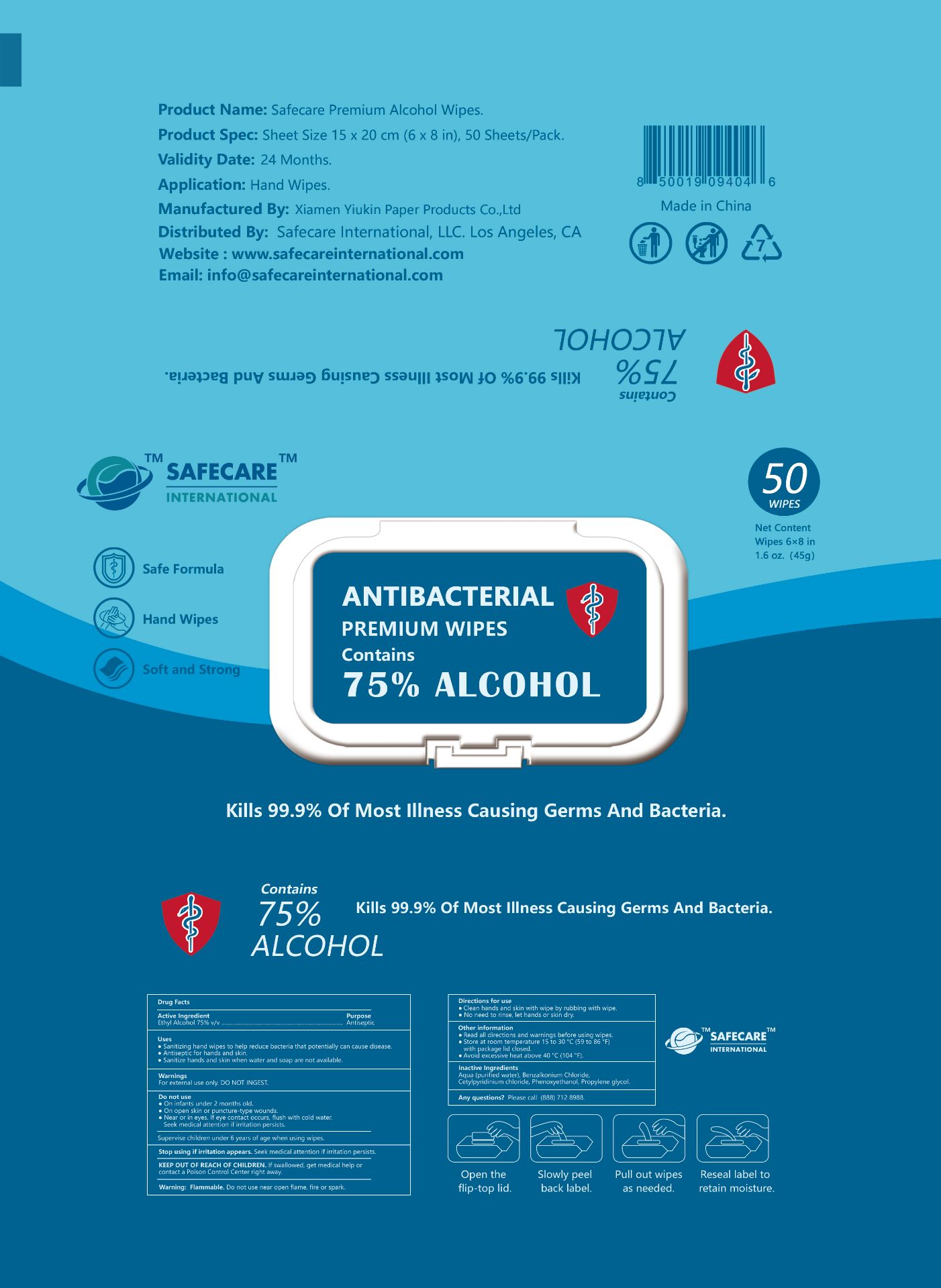
Package Label - Principal Display Panel
50 PCS NDC: 79288-012-01
SAFECARE
INTERNATIONAL
Safe Formula
Hand Wipes
Soft and Strong
ANTIBACERIAL
PREMIUM WIPES
Contains
75% ALCOHOL
50 WIPES
Net Content
Wipes 6 x 8 in
1.6 oz (45g)
Kills 99.9% Of Most Illness Causing Germs And Bacteria.
100 PCS NDC: 79288-012-02
SAFECARE
INTERNATIONAL
Safe Formula
Hand Wipes
Soft and Strong
PREMIUM
SANTIZING WIPES
Kills 99.9% Of Most Illness Causing Germs And Bacteria.
Contains
75% ALCOHOL
100 WIPES
6 inch x 8 inch

160 PCS NDC: 79288-012-03
SAFECARE
INTERNATIONAL
Safe Formula
Hand Wipes
Soft and Strong
PREMIUM
SANTIZING WIPES
Kills 99.9% Of Most Illness Causing Germs And Bacteria.
Contains
75% ALCOHOL
160 WIPES
6 inch x 8 inch

| SAFECARE PREMIUM SANITIZING ALCOHOL WIPES
alcohol cloth |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Xiamen Yiukin Paper Products Co.,ltd (526049172) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Xiamen Yiukin Paper Products Co.,ltd | 526049172 | manufacture(79288-012) , label(79288-012) | |