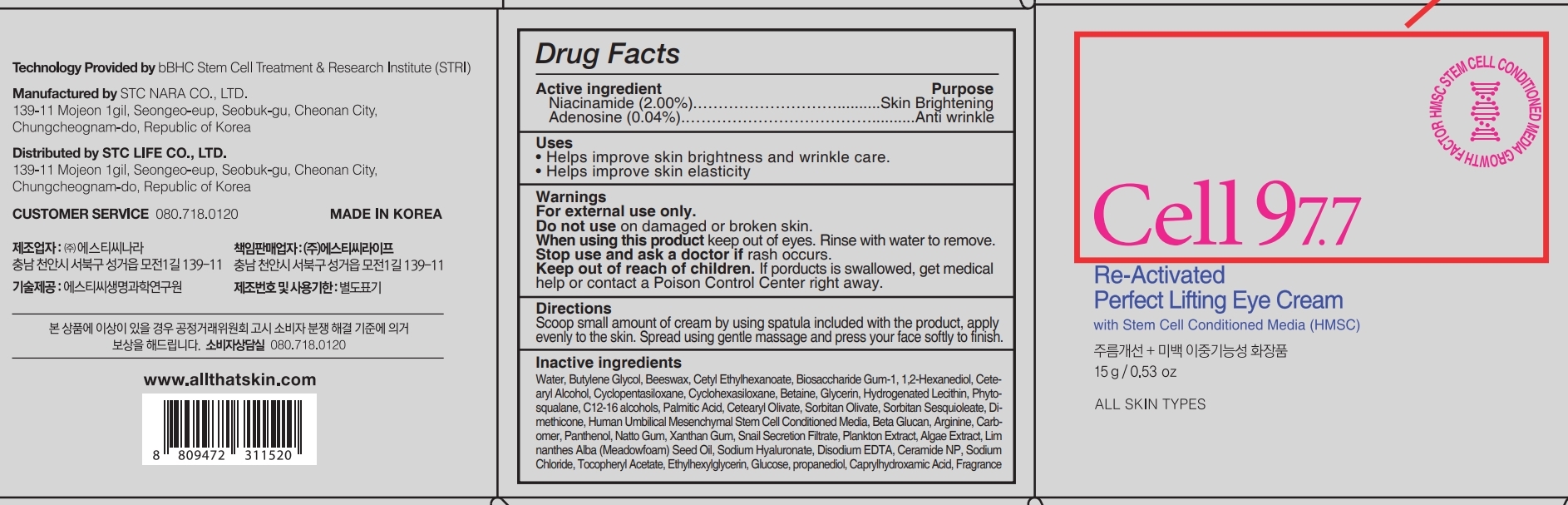
CELL 97.7 RE-ACTIVATED PERFECT LIFTING EYE Cream by BBHC CO., LTD / STC Nara Co., Ltd 76731-207
CELL 97.7 RE-ACTIVATED PERFECT LIFTING EYE Cream by
Drug Labeling and Warnings
CELL 97.7 RE-ACTIVATED PERFECT LIFTING EYE Cream by is a Otc medication manufactured, distributed, or labeled by BBHC CO., LTD, STC Nara Co., Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CELL 97.7 RE-ACTIVATED PERFECT LIFTING EYE CREAM- niacinamide, adenosine cream
BBHC CO., LTD
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
76731-207
Warnings
For external use only.
Do not use on damaged or broken skin.
When using this product keep out of eyes. Rinse with water to remove.
Keep out of reach of children.
If porducts is swallowed, get medical help or contact a Poison Control Center right away.
Directions
Scoop small amount of cream by using spatula included with the product, apply evenly to the skin. Spread using gentle massage and press your face softly to finish.
Inactive ingredients
Water, Butylene Glycol, Beeswax, Cetyl Ethylhexanoate, Biosaccharide Gum-1, 1,2-Hexanediol, Cetearyl Alcohol, Cyclopentasiloxane, Cyclohexasiloxane, Betaine, Glycerin, Hydrogenated Lecithin, Phytosqualane, C12-16 alcohols, Palmitic Acid, Cetearyl Olivate, Sorbitan Olivate, Sorbitan Sesquioleate, Dimethicone, Human Umbilical Mesenchymal Stem Cell Conditioned Media, Beta Glucan, Arginine, Carbomer, Panthenol, Natto Gum, Xanthan Gum, Snail Secretion Filtrate, Plankton Extract, Algae Extract, Lim nanthes Alba (Meadowfoam) Seed Oil, Sodium Hyaluronate, Disodium EDTA, Ceramide NP, Sodium Chloride, Tocopheryl Acetate, Ethylhexylglycerin, Glucose, propanediol, Caprylhydroxamic Acid, Fragrance
| CELL 97.7 RE-ACTIVATED PERFECT LIFTING EYE CREAM
niacinamide, adenosine cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - BBHC CO., LTD (689522401) |
| Registrant - STC Nara Co., Ltd (689135085) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| STC Nara Co., Ltd | 689135085 | manufacture(76731-207) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.