SYALLGEN- penicillium glabrum tablet
SyAllgen by
Drug Labeling and Warnings
SyAllgen by is a Homeopathic medication manufactured, distributed, or labeled by Syntrion GmbH. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- SPL UNCLASSIFIED SECTION
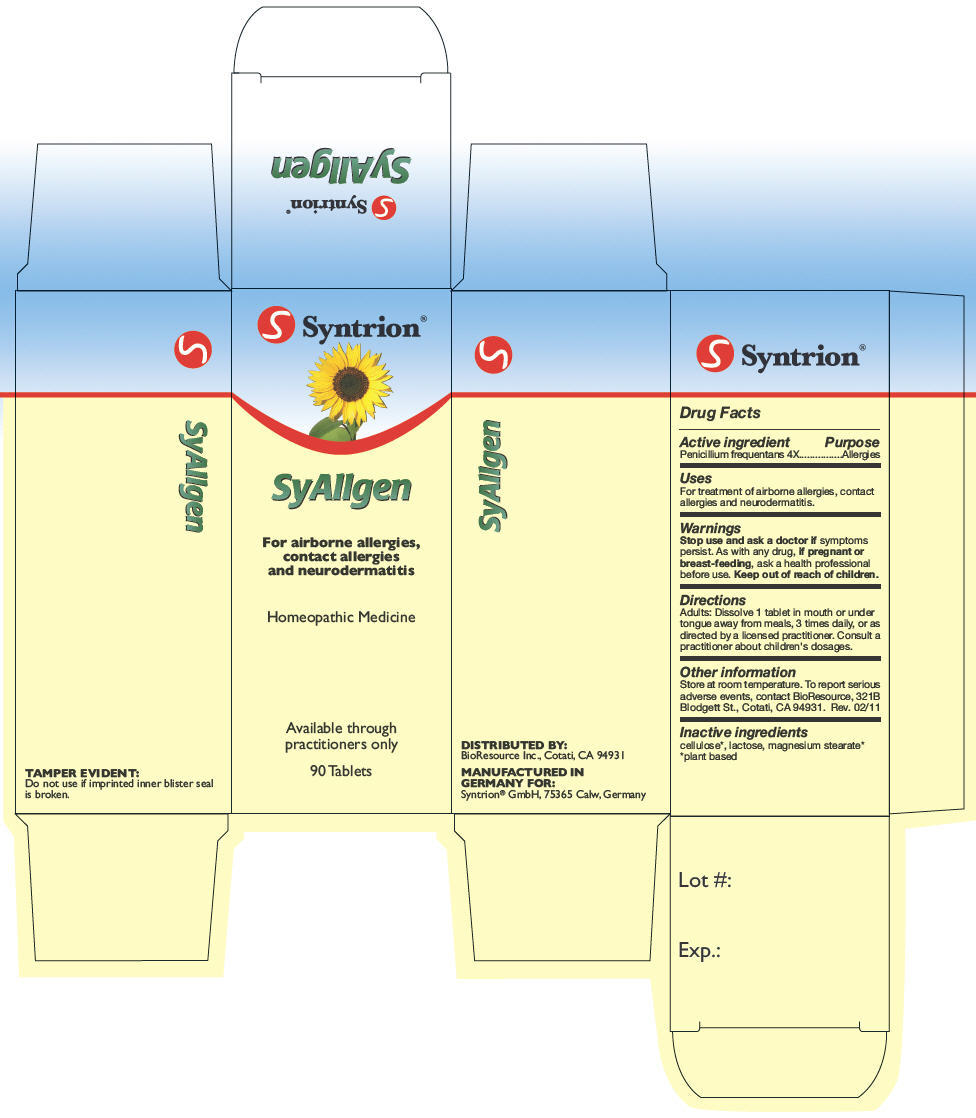
- PRINCIPAL DISPLAY PANEL - 90 Tablet Carton
-
INGREDIENTS AND APPEARANCE
SYALLGEN
penicillium glabrum tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 10424-156 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Penicillium Glabrum (UNII: 2IN23YUE0I) (Penicillium Glabrum - UNII:2IN23YUE0I) Penicillium Glabrum 4 [hp_X] Inactive Ingredients Ingredient Name Strength powdered cellulose (UNII: SMD1X3XO9M) lactose (UNII: J2B2A4N98G) magnesium stearate (UNII: 70097M6I30) Product Characteristics Color WHITE Score no score Shape ROUND (planar on both sides, and with facet) Size 7mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 10424-156-40 1 in 1 CARTON 1 90 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED HOMEOPATHIC 05/18/2006 Labeler - Syntrion GmbH (331883145) Establishment Name Address ID/FEI Business Operations Syntrion GmbH 331883145 MANUFACTURE
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.