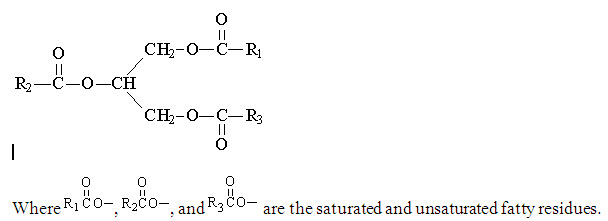CLINOLIPID- olive oil and soybean oil injection, emulsion
Clinolipid by
Drug Labeling and Warnings
Clinolipid by is a Prescription medication manufactured, distributed, or labeled by Baxter Healthcare Corporation, Baxter SA, Baxter R&D Europe SPRL. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CLINOLIPID safely and effectively. See full prescribing information for CLINOLIPID.
CLINOLIPID (lipid injectable emulsion), for intravenous use
Initial U.S. Approval: 1975WARNING: DEATH IN PRETERM INFANTS
See full prescribing information for complete boxed warning
- Deaths in preterm infants have been reported in literature. (5.1, 8.4)
- Autopsy findings included intravascular fat accumulation in the lungs. (5.1, 8.4)
- Preterm and low birth weight infants have poor clearance of intravenous lipid emulsion and increased free fatty acid plasma levels following lipid emulsion infusion. (5.1, 8.4)
INDICATIONS AND USAGE
CLINOLIPID is indicated in adults for parenteral nutrition providing a source of calories and essential fatty acids when oral or enteral nutrition is not possible, insufficient, or contraindicated. (1)
Limitations of Use:- CLINOLIPID is not indicated for use in pediatric patients because there is insufficient data to demonstrate that CLINOLIPID provides sufficient amounts of essential fatty acids in this population (1, 8.4)
- The omega-3: omega-6 fatty acid ratio in CLINOLIPID has not been shown to improve clinical outcomes compared to other intravenous lipid emulsions (1)
DOSAGE AND ADMINISTRATION
- Use a 1.2 micron in-line filter when administering to a patient (2.1)
- See full prescribing information for administration and admixing instructions (2.2, 2.3)
- CLINOLIPID is intended for intravenous infusion. (2.4)
- The recommended dose depends on energy expenditure, clinical status, body weight, tolerance, ability to metabolize and consideration of additional energy given to the patient. The usual daily lipid dosage in adults is 1 to 1.5 g/kg/day and should not exceed 2.5 g/kg/day. (2.4)
DOSAGE FORMS AND STRENGTHS
CLINOLIPID 20% is a lipid injectable emulsion. The lipid content is 0.2 grams/mL in 100 mL, 250 mL, 500 mL, and 1000 mL. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Preterm infants have poor clearance of intravenous lipid emulsion. (5.1)
- Monitor for signs or symptoms of hypersensitivity reactions. (5.2)
- Monitor for signs and symptoms of infection, fat overload, and refeeding complications. (5.3, 5.4, 5.5)
- Frequent clinical and laboratory determinations are necessary. (5.6)
ADVERSE REACTIONS
The most common (5%) adverse drug reactions from clinical trials were nausea and vomiting, hyperlipidemia, hyperglycemia, hypoproteinemia and abnormal liver function tests. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Baxter Healthcare at 1-866-888-2472 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatchDRUG INTERACTIONS
The anticoagulant activity of coumarin derivatives, including warfarin, may be counteracted. (7)
USE IN SPECIFIC POPULATIONS
Hepatic Impaired: Use with caution in patients with preexisting liver disease or liver insufficiency. (8.5)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2016
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: DEATH IN PRETERM INFANTS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Use of an Inline Filter When Administering CLINOLIPID to a Patient
2.2 Important Administration Instructions
2.3 Admixing Guidelines
2.4 Dosing Considerations
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Death in Preterm Infants
5.2 Hypersensitivity Reactions
5.3 Infections
5.4 Fat Overload Syndrome
5.5 Refeeding Syndrome
5.6 Monitoring/Laboratory Tests
5.7 Interference with Laboratory Tests
5.8 Aluminum Toxicity
5.9 Risk of Parenteral Nutrition Associated Liver Disease
5.10 Hypertriglyceridemia
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post-marketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: DEATH IN PRETERM INFANTS
Deaths in preterm infants after infusion of intravenous lipid emulsions have been reported in the medical literature.
Autopsy findings included intravascular fat accumulation in the lungs.
Preterm infants and low birth weight infants have poor clearance of intravenous lipid emulsion and increased free fatty acid plasma levels following lipid emulsion infusion.
[See Warnings and Precautions(5.1)and Use in Specific Populations(8.4)]
-
1 INDICATIONS AND USAGE
CLINOLIPID is indicated in adults for providing a source of calories and essential fatty acids for parenteral nutrition when oral or enteral nutrition is not possible, insufficient, or contraindicated.
Limitations of Use
CLINOLIPID is not indicated for use in pediatric patients because there is insufficient data to demonstrate that CLINOLIPID provides sufficient amounts of essential fatty acids in this population. [See Use in Specific Populations (8.4)]
The omega-3: omega-6 fatty acid ratio in CLINOLIPID has not been shown to improve clinical outcomes compared to other intravenous lipid emulsions. [See Clinical Studies (14)]
-
2 DOSAGE AND ADMINISTRATION
2.1 Use of an Inline Filter When Administering CLINOLIPID to a Patient
Fragments of the administration port membrane could be dislodged into the bag after spiking. Use a 1.2 micron in-line filter during administration of CLINOLIPID (alone or as part of an admixture) to remove particulate matter or micro-precipitate contamination during administration of a lipid injection (alone or as part of an admixture). Particulate matter greater than 5 microns has the capability of obstructing blood flow through capillaries, which could lead to embolism and vascular occlusion. Do not use filters of less than 1.2 micron pore size with lipid emulsions.
2.2 Important Administration Instructions
Before opening the overwrap, check the color of the oxygen indicator. Compare color of the indicator to the reference color printed next to the OK symbol depicted in the printed area of the indicator label. Do not use the product if the color of the oxygen absorber/indicator does not correspond to the reference color printed next to the OK symbol.
After opening the bag, use the contents immediately and discard unused portion.
Visually inspect that the emulsion is a homogeneous liquid with a milky appearance. Inspect for particulate matter and discoloration prior to administration, whenever solution and container permit.
When Administering CLINOLIPID to a Patient:
Do not connect flexible bags in series to avoid air embolism due to possible residual gas contained in the primary bag.
Air embolism can result if residual gas in the bag is not fully evacuated prior to administration if the flexible bag is pressurized to increase flow rates.
Do not use vented administration sets with the vent in the open position. This can result in air embolism.
If CLINOLIPID is mixed with dextrose and/or amino acid solutions, check the compatibility before administration by inspecting the mixture closely for the presence of precipitates. Formation of precipitates could result in vascular occlusion.
When infused alone, CLINOLIPID can be administered via central or peripheral vein. When administered with dextrose and amino acids, the choice of a central or peripheral venous route should depending on the osmolarity of the final infusate.
Do not use administration sets and lines that contain di-2-ehtylhexyl phthalate (DEHP).
Use only a 1.2 micron pore size in-line filter to administer CLINOLIPID. DO NOT use any size less than 1.2 micron pore size in-line filter [see Dosage and Administration (2.1)].
2.3 Admixing Guidelines
When Admixing CLINOLIPID in the Pharmacy:
Prepare the admixture using strict aseptic techniques to avoid microbial contamination.
Do not add additives directly to CLINOLIPID. Do not add CLINOLIPID to the total parenteral nutrition container first; destabilization of the lipid may occur from such an admixture.
Do not use the EXACTAMIX Inlet REF 173 (H938173) with an EXACTAMIX compounder to transfer CLINOLIPID. This inlet spike has been associated with dislodgement of the administration port membrane into the CLINOLIPID bag. Use of EXACTAMIX Inlet REF 174 (H938174) is recommended.
The following proper mixing sequence must be followed to minimize pH related problems by ensuring that typically acidic Dextrose Injections are not mixed with lipid emulsions alone:
- 1. Transfer Dextrose Injection to the Total Parenteral Nutrition Admixture Container
- 2. Transfer Amino Acid Injection
- 3. Transfer Lipid Emulsion
Amino Acid Injection, Dextrose Injection and Lipid Emulsions may be simultaneously transferred to the admixture container. Use gentle agitation during admixing to minimize localized concentration effects; shake bags gently after each addition.
The prime destabilizers of emulsions are excessive acidity (such as a pH below 5) and inappropriate electrolyte content. Give careful consideration to additions of divalent cations (Ca++ and Mg++), which have been shown to cause emulsion instability. Amino acid solutions exert buffering effects that protect the emulsion.
Inspect the admixture closely for separation of the emulsion. This can be visibly identified by a yellowish streaking or the accumulation of yellowish droplets in the admixed emulsion. The admixture should also be examined for particulates. Discard the admixture if any of the above is observed.
2.4 Dosing Considerations
The dosing of CLINOLIPID depends on energy expenditure, the patient’s clinical status, body weight, tolerance, and ability to metabolize CLINOLIPID, as well as additional energy given orally/enterally to the patient. For complete parenteral nutrition, concomitant supplementation with amino acids, carbohydrates, electrolytes, vitamins, and trace elements is necessary.
Prior to administration of CLINOLIPID, correct severe water and electrolyte disorders, severe fluid overload states, and severe metabolic disorders. Before starting the infusion, obtain serum triglyceride levels to establish the baseline value. In patients with elevated triglyceride levels, initiate CLINOLIPID injection at a lower dose, and advance in smaller increments, checking the triglyceride levels prior to each adjustment.
Adjust the administration flow rate by taking into account the dose being administered, the daily volume intake, and the duration of the infusion [see Overdosage (10)].
The recommended duration of infusion for a parenteral nutrition bag is between 12 and 24 hours, depending on the clinical situation. Treatment with parenteral nutrition may be continued for as long as is required by the patient’s condition.
The maximum daily dose of CLINOLIPID should be based on individual total nutritional requirements and patient tolerance. The usual lipid dosage is 1 to 1.5 g/kg/day (equal to 5 to 7.5 mL/kg/day of CLINOLIPID 20%) 1. The daily dose should not exceed 2.5 g/kg/day. The initial infusion rate should not exceed 0.1 g (equal to 0.5 mL) per minute for the first 15 to 30 minutes. If tolerated, gradually increase until reaching the required rate after 30 minutes.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
The use of CLINOLIPID is contraindicated in patients with the following:
- Known hypersensitivity to egg or soybean proteins or to any of the ingredients, including excipients.
- Severe hyperlipidemia (serum triglyceride concentrations above 1000 mg/dL) or severe disorders of lipid metabolism characterized by hypertriglyceridemia.
-
5 WARNINGS AND PRECAUTIONS
5.1 Death in Preterm Infants
Deaths in preterm infants after infusion of intravenous lipid emulsions have been reported1. Autopsy findings included intravascular lipid accumulation in the lungs.
Preterm and small for gestational age infants have poor clearance of intravenous lipid emulsion and increased free fatty acid plasma levels following lipid emulsion infusion.
The safe and effective use of CLINOLIPID in pediatric patients, including preterm infants, has not been established. CLINOLIPID is not indicated for and not recommended for use in pediatric patients.
5.2 Hypersensitivity Reactions
Stop infusion immediately and treat patient accordingly if signs or symptoms of a hypersensitivity or allergic reaction develop. Signs or symptoms may include: tachypnea, dyspnea, hypoxia, bronchospasm, tachycardia, hypotension, cyanosis, vomiting, nausea, headache, sweating, dizziness, altered mentation, flushing, rash, urticaria, erythema, pyrexia and chills.
5.3 Infections
Patients who require parenteral nutrition are at high risk of infections due to malnutrition and their underlying disease state.
Infection and sepsis may occur as a result of the use of intravenous catheters to administer parenteral nutrition, poor maintenance of catheters, or immunosuppressive effects of illness, drugs, and parenteral formulations.
Decrease the risk of septic complications with heightened emphasis on aseptic technique in catheter placement and maintenance, as well as aseptic technique in the preparation of the nutritional formula.
Carefully monitor for signs and symptoms (including fever and chills) of early infections, including laboratory test results (including leukocytosis and hyperglycemia) and frequent checks of the parenteral access device.
5.4 Fat Overload Syndrome
Fat overload syndrome is a rare condition that has been reported with intravenous lipid formulations. A reduced or limited ability to metabolize the lipids contained in CLINOLIPID accompanied by prolonged plasma clearance may result in a syndrome characterized by a sudden deterioration in the patient's condition accompanied by fever, anemia, leukopenia, thrombocytopenia, coagulation disorders, hyperlipidemia, liver fatty infiltration (hepatomegaly), deteriorating liver function, and central nervous system manifestations (e.g., coma). The cause of the fat overload syndrome is unclear. The syndrome is usually reversible when the infusion of the lipid emulsion is stopped. Although it has been most frequently observed when the recommended lipid dose was exceeded, cases have also been described where the lipid formulation was administered according to instructions.
5.5 Refeeding Syndrome
Refeeding severely undernourished patients with parenteral nutrition may result in the refeeding syndrome, characterized by the intracellular shift of potassium, phosphorus, and magnesium as the patient becomes anabolic. Thiamine deficiency and fluid retention may also develop. Carefully monitor severely undernourished patients and slowly increase their nutrient intakes, while avoiding overfeeding, to prevent these complications.
5.6 Monitoring/Laboratory Tests
Routine Monitoring
Monitor fluid status closely in patients with pulmonary edema or heart failure.
Monitor serum triglycerides, fluid and electrolyte status, serum osmolarity, blood glucose, liver and kidney function, and blood count, including platelets and coagulation parameters, throughout treatment.
Essential Fatty Acids
Monitoring patients for signs and symptoms of essential fatty acid deficiency (EFAD) is recommended. Laboratory tests are available to determine serum fatty acids levels. Reference values should be consulted to help determine adequacy of essential fatty acid status. Increasing essential fatty acid intake (enterally or parenterally) is effective in treating and preventing EFAD.
In CLINOLIPID injection, the mean composition of linoleic acid (an omega-6 essential fatty acid) is 35.8 mg/mL (range 27.6 to 44.0 mg/mL) and α-linolenic acid (an omega-3 essential fatty acid) is 4.7 mg/mL (range 1.0 to 8.4 mg/mL). There are insufficient long-term data to determine whether CLINOLIPID 20% can supply essential fatty acids in adequate amounts in patients who may have increased requirements.
5.7 Interference with Laboratory Tests
Content of Vitamin K may counteract anticoagulant activity [see Drug Interactions (7)].
The lipids contained in this emulsion may interfere with the results of certain laboratory tests if the blood sample is taken before the lipids are eliminated from the serum (these are generally eliminated after a period of 5 to 6 hours without receiving lipids).
5.8 Aluminum Toxicity
CLINOLIPID contains no more than 25 mcg/L of aluminum.
The aluminum contained in CLINOLIPID may reach toxic levels with prolonged administration in patients with impaired kidney function. Preterm infants are at greater risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions that contain aluminum.
Patients with impaired kidney function, including preterm infants, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day, accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration of total parenteral nutrition products.
5.9 Risk of Parenteral Nutrition Associated Liver Disease
Parenteral Nutrition Associated Liver Disease (PNALD) has been reported in patients who receive parenteral nutrition for extended periods of time, especially preterm infants, and can present as cholestasis or steatohepatitis. The exact etiology is unknown and is likely multifactorial. Intravenously administered phytosterols (plant sterols) contained in plant-derived lipid formulations have been associated with development of PNALD although a causal relationship has not been clearly established. If CLINOLIPID treated patients develop liver test abnormalities consider discontinuation or dose reduction.
5.10 Hypertriglyceridemia
Reduce dose of CLINOLIPID and monitor serum triglyceride levels in patients with serum triglyceride concentrations above 400 mg/dL to avoid the clinical consequences associated with hypertriglyceridemia. Serum triglyceride levels above 1000 mg/dL have been associated with an increased risk of pancreatitis.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The CLINOLIPID trials had small sample sizes and patients had a variety of underlying medical conditions both between different trials and within the individual trials. Patients had gastrointestinal diseases/dysfunction or were recovering from gastrointestinal or other surgeries, trauma, burns, or were afflicted by other chronic illness. The largest trial (Study 1, 48 subjects) enrolled patients with many different underlying diagnoses. The rates of treatment emergent adverse reactions can therefore not be directly compared to rates observed in the clinical trials of other related products and may not reflect the rates observed in clinical practice.
Commonly observed adverse reactions in 261 adult patients who received CLINOLIPID were nausea and vomiting, hyperlipidemia, hyperglycemia, hypoproteinemia and abnormal liver function tests and occurred in 2 to 10 % of patients. In Study 1 the most common adverse reactions were infectious complications (urinary tract infection, septicemia, and fever of unknown origin), treatment emergent abnormalities on liver/gallbladder ultrasound and abnormalities of serum chemistries, principally, hepatic function tests. Adverse reactions in Study 2 were similar.
Adverse reactions reported with other intravenous lipid emulsions include hyperlipidemia, hypercoagulability, thrombophlebitis, and thrombocytopenia.
Adverse reactions reported in long-term use with other intravenous lipid emulsions include hepatomegaly, jaundice due to central lobular cholestasis, splenomegaly, thrombocytopenia, leukopenia, abnormalities in liver function tests, brown pigmentation of the liver and overloading syndrome (focal seizures, fever, leukocytosis, hepatomegaly, splenomegaly and shock).
6.2 Post-marketing Experience
The following adverse reactions have been identified during use of CLINOLIPID, and listed by MedDRA System Organ Class, then by Preferred Term in order of severity. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal Disorders: Diarrhea
Skin and Subcutaneous Tissue Disorders: Pruritus
Immune System Disorders: Hypersensitivity with the manifestations of rash and dyspnea
Investigations: International normalized ratio (INR) decreased in anticoagulated patients [see Drug Interactions (7)]
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The limited available data on the use of CLINOLIPID in pregnant women are not sufficient to inform a drug-associated risk. However, there are clinical considerations if CLINOLIPID is used in pregnant women [see Clinical Considerations]. Animal reproduction studies have not been conducted with lipid injectable emulsion.
The estimated background risk of major birth defects and miscarriage for the indicated population are unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and15 to 20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo-Fetal Risk:
Severe malnutrition in a pregnant woman is associated with preterm delivery, low birth weight, intrauterine growth restriction, congenital malformations and perinatal mortality. Parenteral nutrition should be considered if a pregnant woman’s nutritional requirements cannot be fulfilled by oral or enteral intake. It is not known whether the administration of CLINOLIPID to pregnant women provides adequate essential fatty acids to the developing fetus.8.2 Lactation
Risk Summary
There are no data available to assess the presence of CLINOLIPID and/or its active metabolite(s) in human milk, the effects on the breastfed child or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for CLINOLIPID and any potential adverse effects on the breastfed child from CLINOLIPID or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of CLINOLIPID have not been established in pediatric patients. CLINOLIPID is not indicated for use in pediatric patients. Pediatric studies did not establish that CLINOLIPID provides sufficient amounts of essential fatty acids (EFA) in pediatric patients. Pediatric patients may be particularly vulnerable to neurologic complications due to EFA deficiency if adequate amounts of EFA are not provided.
Deaths in preterm infants after infusion of intravenous lipid emulsion have been reported [see Warnings and Precautions (5.1)]. Patients, particularly preterm infants, are at risk for aluminum toxicity [see Warnings and Precautions (5.8)]. Patients, including pediatric patients, may be at risk for PNALD [see Warnings and Precautions (5.9)]. In clinical trials of a pure soybean oil based intravenous lipid emulsion product, thrombocytopenia in neonates occurred (less than 1%).
8.5 Geriatric Use
Of the total number of subjects in clinical studies of CLINOLIPID, 21% were 65 and over, while 10% were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Hepatic Impairment
Parenteral nutrition should be used with caution in patients with hepatic impairment. Hepatobiliary disorders are known to develop in some patients without preexisting liver disease who receive parenteral nutrition, including cholestasis, hepatic steatosis, fibrosis and cirrhosis (parenteral nutrition associated liver disease), possibly leading to hepatic failure. Cholecystitis and cholelithiasis have also been observed. The etiology of these disorders is thought to be multifactorial and may differ between patients.
Monitor liver function parameters closely. Patients developing signs of hepatobiliary disorders should be assessed early by a clinician knowledgeable in liver diseases in order to identify causative and contributory factors, and possible therapeutic and prophylactic interventions.
-
10 OVERDOSAGE
In the event of overdose, fat overload syndrome may result [see Warnings and Precautions (5.4)]. Stop the infusion to allow lipids to clear from serum. The effects are usually reversible after the lipid infusion is stopped. If medically appropriate, further intervention may be indicated. The lipid administered and fatty acids produced are not dialyzable.
-
11 DESCRIPTION
CLINOLIPID lipid injectable emulsion, USP is a sterile, non-pyrogenic lipid emulsion for intravenous infusion. CLINOLIPID is a lipid emulsion containing a mixture of refined olive oil and refined soybean oil in an approximate ratio of 4:1 (olive:soy). The lipid content is 0.2 g/mL. In CLINOLIPID, the mean composition of linoleic acid (an omega-6 essential fatty acid) is 35.8 mg/mL (range 27.6 to 44.0 mg/mL) and α-linolenic acid (an omega-3 essential fatty acid) is 4.7 mg/mL (range 1.0 to 8.4 mg/mL). The phospholipids provide 470 milligrams or 15 mmol of phosphorus per liter.
The total energy content, including fat, phospholipids and glycerin is 2000 kcal/L.Each 100 mL of CLINOLIPID 20% contains approximately 16 g of Olive Oil NF and 4 g of Soybean Oil USP, 1.2 g Egg Phospholipids NF, 2.25 g Glycerin USP, 0.03 g Sodium Oleate, and Water for Injection USP. Sodium Hydroxide NF for pH adjustment, pH: 6.0 to 9.0.
The olive and soybean oils are refined natural products consisting of a mixture of neutral triglycerides of predominantly unsaturated fatty acids with the following structure:
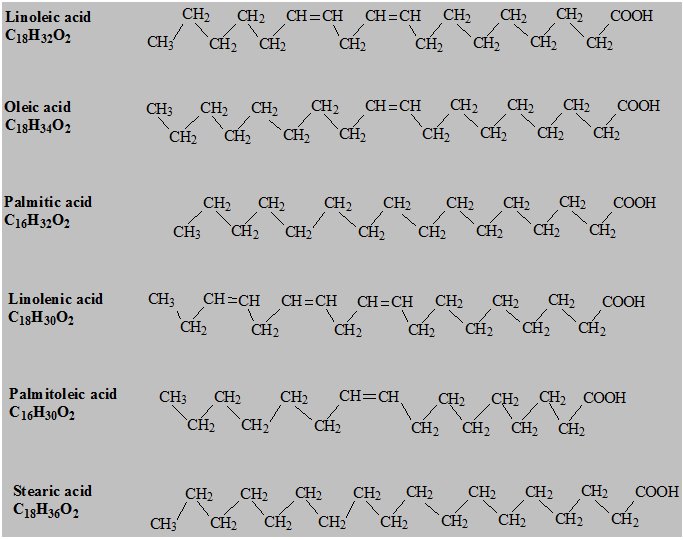
The major component fatty acids are linoleic (13.8 to 22.0%), oleic (44.3 to 79.5%), palmitic (7.6 to 19.3%), linolenic (0.5 to 4.2%), palmitoleic (0.0 to 3.2%) and stearic (0.7 to 5.0%). These fatty acids have the following chemical and structural formulas:
CLINOLIPID has an osmolality of approximately 340 mOsmol/kg water (which represents an osmolarity of 260 mOsmol/liter of emulsion)
CLINOLIPID contains no more than 25 mcg/L of aluminum.
-
12 CLINICAL PHARMACOLOGY
CLINOLIPID administered intravenously provides biologically utilizable source of calories and essential fatty acids.
12.1 Mechanism of Action
Fatty acids serve as an important substrate for energy production. The most common mechanism of action for energy production derived from fatty acid metabolism is beta oxidation. Fatty acids are important for membrane structure and function, precursors for bioactive molecules (such as prostaglandins), and as regulators of gene expression.
12.2 Pharmacodynamics
Infused essential fatty acids are synthesized into higher derivative fatty acids. Olive oil contains significant amounts of alpha-tocopherol that contributes to Vitamin E status.
12.3 Pharmacokinetics
Metabolism and Excretion
The fatty acids, phospholipids, and glycerol found in lipid emulsions are metabolized by cells to carbon dioxide and water. The metabolism of these substances results in the generation of energy in the form of adenosine triphosphate (ATP). Some fatty acids are stored in the body in fat tissue, cell membranes, or as intracellular triglycerides. There is constant turn-over of these tissues, with the result that the lipid components are eventually metabolized to carbon dioxide and water. Carbon dioxide is expired through the lungs. Water is excreted through the kidneys or lost through evaporation/expiration through the skin, lungs, and other tissue surfaces. Some lipids (i.e., phospholipids, cholesterol, and bile acids) are excreted through the biliary system.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies with CLINOLIPID have not been performed to evaluate the carcinogenic potential, mutagenic potential, or effects on fertility.
13.2 Animal Toxicology and/or Pharmacology
CLINOLIPID was evaluated in toxicity studies conducted in rats and dogs for up to 3 months. The principle signs of toxicity noted in the 3-month studies were:
- Slight hemolytic anemia at 12 g/kg/day in rats and at 6 g/kg/day in dogs. These doses in rats and dogs are 4.8 and 2.4 times higher, respectively, than the recommended adult dose (2.5 g/kg/day) of CLINOLIPID.
- Dose-dependent decrease in urea levels in rats at 6 and 12 g/kg/day dose levels and in dogs at 3, 4.5 and 6 g/kg/day dose levels associated with decreased feed consumption.
- Hypercholesterolemia in dogs at 3, 4.5 and 6 g/kg/day dose levels.
- Hepatic pathology of lipid and pigmentary overload in male and female rats at 3, 6 and 12 g/kg/day dose levels and brownish-yellow pigmentation in vacuolated Kupffer cells in male and female dogs at 3, 4.5 and 6 g/kg/day dose levels with hepatocyte vacuolation in male dogs at 6 g/kg/day and female dogs at 4.5 and 6 g/kg/day dose levels.
- Splenic pigmentation and vacuolization in rats at 3, 6 and 12 g/kg/day dose levels, and dogs in 4.5 and 6 g/kg/day dose levels.
At doses of 3 g/kg/day, slight lipid and pigmentary overload of the liver and vacuolization of Kupffer cells were observed in rats and dogs. At a dose of 12 g/kg/day in rats, hepatocellular vacuolation, granulomatous inflammation of the liver, hepatocellular necrosis and hemosiderosis of the liver and lipid deposits and splenic hemosiderosis, were observed. In dogs, at a dose of 6 g/kg/day, brownish-yellow pigmentation in the Kupffer cells of liver and spleen, hyperplasia of vacuolated Kupffer cells, hepatocyte vacuolization, a slight increase in the number of lipid storage cells (Ito cells) in the liver and macrophage vacuolization of the spleen were observed.
-
14 CLINICAL STUDIES
Two clinical trials (Study 1 and Study 2) in adults compared CLINOLIPID to a pure soybean oil based intravenous lipid emulsion. Although Study 1 and Study 2 were not adequately designed to demonstrate noninferiority of CLINOLIPID to the soybean oil comparator, they support CLINOLIPID injection as a source of calories and essential fatty acids in adults. The lipid dosage was variable in Studies 1 and 2 and adjusted to the patient’s nutritional requirements.
Study 1 was a randomized, open-label, multicenter study. Forty eight (48) patients, aged 17 to 75 years, requiring ≥15 days (mean 22 days) exclusive parenteral nutrition (TPN) were enrolled and randomized to either CLINOLIPID or a pure soybean oil based intravenous lipid emulsion. Nutritional efficacy was assessed by anthropometric indices (body weight, arm circumference, skin-fold thickness), biomarkers of protein metabolism (total protein, albumin) and lipid metabolism. Anthropometric criteria (body weight, arm circumference, and skin fold thickness) were comparable for both groups. Mean total serum protein and albumin increased similarly in both groups.
Study 2 was a randomized, open label multicenter study that enrolled 22 patients aged 32-81 years who required long-term parenteral nutrition. Twelve patients received CLINOLIPID for a mean of 202 days (range 24 to 408 days) and 10 patients received the comparator lipid for a mean of 145 days (range 29-394 days). The two groups had similar outcomes for weight, weight loss, mid-arm circumference and triceps skinfold thickness.
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
CLINOLIPID lipid injectable emulsion, USP is supplied in single-dose CLARITY polyolefin bags as follows:
Container Size
NDC Number (1 Bag)
NDC Number (Shelf Pack)
100 mL
0338-9540-01
0338-9540-05 (15 pack)
250 mL
0338-9540-02
0338-9540-06 (10 pack)
500 mL
0338-9540-03
0338-9540-07 (12 pack)
1000 mL
0338-9540-04
0338-9540-08 (6 pack)
The CLARITY Container is a lipid-compatible plastic container (PL 2401-1). The bag is packaged in an oxygen barrier overpouch, which contains an oxygen absorber / oxygen indicator sachet.
CLINOLIPID should be stored at 20 to 25 °C (68 to 77 °F). Excursion permitted between 15 to 30 °C (59 to 86 °F). See USP Controlled Room Temperature. Protect from freezing. Avoid excessive heat. Store in overpouch until ready to use.
-
17 PATIENT COUNSELING INFORMATION
To ensure the safe and effective use of CLINOLIPID, this information should be discussed with the patient.
Inform patients of the following:
- Deaths in preterm infants after infusion of intravenous lipid emulsions such as CLINOLIPID have been reported.
- CLINOLIPID is given by infusion through a central or peripheral vein.
- Laboratory monitoring throughout treatment may be necessary.
- Allergic reactions to the lipid emulsion may occur.
- Risk of infection and sepsis associated with formulations administered intravenously.
- Fat overload syndrome can be caused by accumulation of fat in tissues, which may result in adverse effects.
- CLINOLIPID may cause adverse reactions such as nausea and vomiting, excess fat (lipids) in the blood, high blood sugar, low levels of protein in the blood and abnormal liver function tests.
Should patients self-administer CLINOLIPID at home, patients should also be instructed to:
- Do not deviate from the administration instructions given by the health provider.
- Inspect the bag visually for particulate matter and if the lipid emulsion is an evenly distributed liquid with a milky appearance with no visible oil droplets at the surface prior to administration.
- Ensure that there is a 1.2 micron in-line filter in place prior to and during administration.
- Inform their physicians about any changes in prescription or over-the-counter medications and supplements.
- Have periodic laboratory tests and routinely follow up with their healthcare provider.
- Any remaining product from partially used bag must be discarded.
- Contact their healthcare provider should any signs of injection site infection, inflammation extending from the injection site, or new-onset allergic reaction appear.
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Baxter, Clarity, Clinolipid and Exactamix are trademarks of Baxter International Inc., or its subsidiaries.
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
1000 mL
EADB9524 NDC: 0338-9540-04
Baxter Logo
Clinolipid
(Lipid Injectable Emulsion, USP) 20%
200 g/1000 mL (0.2 g/mL)Intravenous use only
1000 mL sterile single dose container
Energy Content 2000 kcal/LEach 100 mL contains approximately 16 g of Olive Oil NF
and 4 g of Soybean Oil USP, 1.2 g Egg Phospholipids NF,
2.25 g Glycerin USP, 0.03 g Sodium Oleate, Water for
Injection USP and Sodium Hydroxide NF for pH
adjustment pH 6.0–9.0 Osmolarity 260 mOsmol/L (calc)Cautions Use only if the color of the oxygen indicator is
within allowable range Do not use unless emulsion has
homogeneous milky appearanceDo not add supplemental medications Must not be used
in series connectionsUsual Dosage See package insert
Store at 20 to 25 °C (68 to 77 °F)
Excursion permitted between 15 to 30 °C
(59 to 86 °F)
See USP Controlled Room Temperature
Protect from freezing
Do not use beyond 24 hours once opened;
discard unused portion after 24 hours
Rx OnlyBaxter Healthcare Corporation
BE-35-02-746
EXP LOT
1000
750
500
250
100
-
INGREDIENTS AND APPEARANCE
CLINOLIPID
olive oil and soybean oil injection, emulsionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0338-9540 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OLIVE OIL (UNII: 6UYK2W1W1E) (OLIVE OIL - UNII:6UYK2W1W1E) OLIVE OIL 16 g in 100 mL SOYBEAN OIL (UNII: 241ATL177A) (SOYBEAN OIL - UNII:241ATL177A) SOYBEAN OIL 4 g in 100 mL Inactive Ingredients Ingredient Name Strength EGG PHOSPHOLIPIDS (UNII: 1Z74184RGV) 1.20 g in 100 mL GLYCERIN (UNII: PDC6A3C0OX) 2.25 g in 100 mL SODIUM OLEATE (UNII: 399SL044HN) 0.03 g in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0338-9540-08 6 in 1 CARTON 01/01/2016 1 NDC: 0338-9540-04 1000 mL in 1 BAG; Type 0: Not a Combination Product 2 NDC: 0338-9540-07 12 in 1 CARTON 01/01/2016 2 NDC: 0338-9540-03 500 mL in 1 BAG; Type 0: Not a Combination Product 3 NDC: 0338-9540-06 10 in 1 CARTON 01/01/2016 3 NDC: 0338-9540-02 250 mL in 1 BAG; Type 0: Not a Combination Product 4 NDC: 0338-9540-05 15 in 1 CARTON 01/01/2016 4 NDC: 0338-9540-01 100 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA204508 01/01/2016 Labeler - Baxter Healthcare Corporation (005083209) Establishment Name Address ID/FEI Business Operations Baxter SA 370353835 MANUFACTURE(0338-9540) , PACK(0338-9540) , ANALYSIS(0338-9540) , STERILIZE(0338-9540) , LABEL(0338-9540) Establishment Name Address ID/FEI Business Operations Baxter R&D Europe SPRL 400409112 ANALYSIS(0338-9540)
Trademark Results [Clinolipid]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CLINOLIPID 88314985 not registered Live/Pending |
Baxter International Inc. 2019-02-25 |
 CLINOLIPID 85885747 not registered Dead/Abandoned |
BAXTER INTERNATIONAL INC. 2013-03-25 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.