ACTICON- dexbrompheniramine maleate, pseudoephedrine hydrochloride solution
ACTICON by
Drug Labeling and Warnings
ACTICON by is a Otc medication manufactured, distributed, or labeled by ACTIPHARMA, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
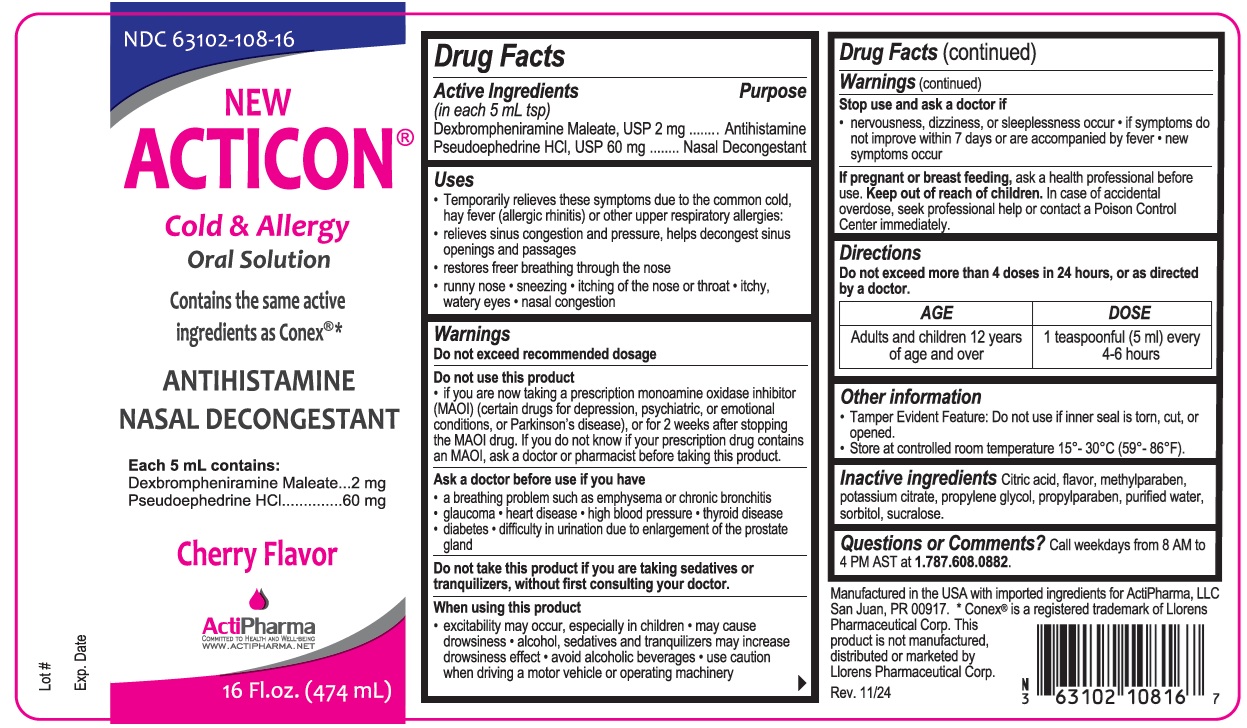
- Drug Facts
- Active Ingredients (in each 5 mL tsp)
- Purpose
-
Uses
Temporarily relieves these symptoms due to the common cold, hay fever (allergic rhinitis) or other upper respiratory allergies:
relieves sinus congestion and pressure, helps decongest sinus openings and passages
restores freer breathing through the nose
runny nose sneezing itching of the nose or throat itchy, watery eyes nasal congestion -
Warnings
Do not exceed recommended dosage
Do not use this product
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
a breathing problem such as emphysema or chronic bronchitis glaucoma heart disease high blood pressure thyroid disease diabetes difficulty in urination due to enlargement of the prostate gland
Do not take this product if you are taking sedatives or tranquilizers, without first consulting your doctor.
When using this product
excitability may occur, especially in children may cause drowsiness alcohol, sedatives and tranquilizers may increase drowsiness effect avoid alcoholic beverages use caution when driving a motor vehicle or operating machineryStop use and ask a doctor if
nervousness, dizziness, or sleeplessness occur if symptoms do not improve within 7 days or are accompanied by fever new symptoms occurIf pregnant or breast feeding, ask a health professional before use.
- Directions
- Other information
- Inactive ingredients
- Questions or Comments?
-
SPL UNCLASSIFIED SECTION
Contains the same active ingredients as Conex®*
Cherry Flavor
ActiPharma
COMMITTED TO HEALTH AND WELL-BEING
WWW.ACTIPHARMA.NETManufactured in the USA with imported ingredients for ActiPharma, LLC San Juan, PR 00917. * Conex® is a registered trademark of Llorens Pharmaceutical Corp. This product is not manufactured, distributed or marketed by Llorens Pharmaceutical Corp.
- Packaging
-
INGREDIENTS AND APPEARANCE
ACTICON
dexbrompheniramine maleate, pseudoephedrine hydrochloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 63102-108 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXBROMPHENIRAMINE MALEATE (UNII: BPA9UT29BS) (DEXBROMPHENIRAMINE - UNII:75T64B71RP) DEXBROMPHENIRAMINE MALEATE 2 mg in 5 mL PSEUDOEPHEDRINE HYDROCHLORIDE (UNII: 6V9V2RYJ8N) (PSEUDOEPHEDRINE - UNII:7CUC9DDI9F) PSEUDOEPHEDRINE HYDROCHLORIDE 60 mg in 5 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) METHYLPARABEN (UNII: A2I8C7HI9T) POTASSIUM CITRATE (UNII: EE90ONI6FF) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63102-108-16 474 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 02/15/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 02/15/2022 Labeler - ACTIPHARMA, LLC (079340948)
Trademark Results [ACTICON]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ACTICON 87612699 not registered Dead/Abandoned |
Acticon LLC 2017-09-18 |
 ACTICON 75894097 not registered Dead/Abandoned |
General Patent Corporation International 2000-01-09 |
 ACTICON 75670181 2432215 Live/Registered |
AMS Research Corporation 1999-03-29 |
 ACTICON 74396639 1879062 Dead/Cancelled |
ROBERT LEHRER ASSOCIATES, INC. 1993-06-01 |
 ACTICON 73607212 1427338 Dead/Cancelled |
RAPITECH SYSTEMS INC. 1986-06-30 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.