LIVRELIEF PAIN RELIEF- capsaicin cream
LivRelief by
Drug Labeling and Warnings
LivRelief by is a Otc medication manufactured, distributed, or labeled by LivCorp Inc., Les Emballages Facoteck Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
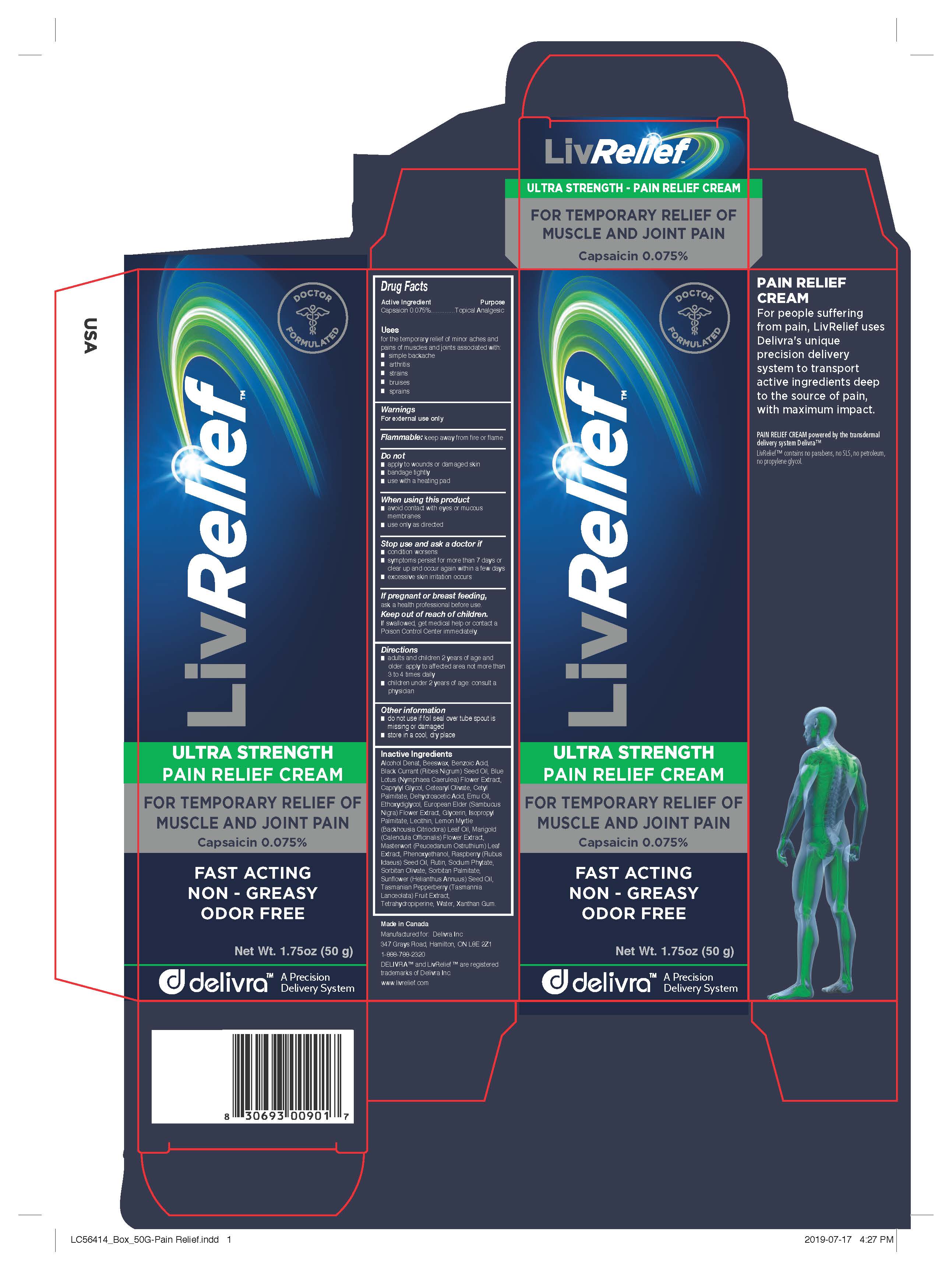
- Active Ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
Inactive Ingredients
Alcohol Denat, Beeswax, Benzoic Acid, Black Currant (Ribes Nigrum) Seed Oil, Blue Lotus (Nymphaea Caerulea) Flower Extract,
Caprylyl Glycol, Cetearyl Olivate, Cetyl Palmitate, Dehydroacetic Acid, Emu Oil, Ethoxydiglycol, European Elder (Sambucus
Nigra) Flower Extract, Glycerin, Isopropyl Palmitate, Lecithin, Lemon Myrtle (Backhousia Citriodora) Leaf Oil, Marigold
(Calendula Officinalis) Flower Extract, Masterwort (Peucedanum Ostruthium) Leaf Extract, Phenoxyethanol, Raspberry (Rubus
Idaeus) Seed Oil, Rutin, Sodium Phytate, Sorbitan Olivate, Sorbitan Palmitate, Sunflower (Helianthus Annuus) Seed Oil,
Tasmanian Pepperberry (Tasmannia Lanceolata) Fruit Extract, Tetrahydropiperine, Water, Xanthan Gum. - Manufactured for LivCorp Inc.
-
Side Panel
PAIN RELIEF CREAM
For people suffering from pain, LivRelief uses Delivra's unique precision delivery system to transport active ingredients deep to the source of pain, with maximum impact.
PAIN RELIEF CREAM powered by the transdermal delivery system Delivra™LivRelief™ contains no parabens, no SLS, no petroleum, no propylene glycol.
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
LIVRELIEF PAIN RELIEF
capsaicin creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 42903-008 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAPSAICIN (UNII: S07O44R1ZM) (CAPSAICIN - UNII:S07O44R1ZM) CAPSAICIN 0.0375 g in 50 g Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) BACKHOUSIA CITRIODORA LEAF OIL (UNII: 2UQQ0PI06P) YELLOW WAX (UNII: 2ZA36H0S2V) BENZOIC ACID (UNII: 8SKN0B0MIM) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CETEARYL OLIVATE (UNII: 58B69Q84JO) CETYL PALMITATE (UNII: 5ZA2S6B08X) DEHYDROACETIC ACID (UNII: 2KAG279R6R) EMU OIL (UNII: 344821WD61) RUBUS IDAEUS SEED (UNII: M3CL7US2ZG) GLYCERIN (UNII: PDC6A3C0OX) SUNFLOWER OIL (UNII: 3W1JG795YI) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) NYMPHAEA CAERULEA FLOWER (UNII: S9560USZ74) PEUCEDANUM OSTRUTHIUM LEAF (UNII: 86P27YRR6Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) RIBES NIGRUM SEED OIL (UNII: GKE1188837) RUTIN (UNII: 5G06TVY3R7) SAMBUCUS NIGRA FLOWER (UNII: 07V4DX094T) PHYTATE SODIUM (UNII: 88496G1ERL) SORBITAN OLIVATE (UNII: MDL271E3GR) SORBITAN MONOPALMITATE (UNII: 77K6Z421KU) TASMANNIA LANCEOLATA FRUIT (UNII: PNT2HDL13Q) TETRAHYDROPIPERINE (UNII: 8904DO502T) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42903-008-50 1 in 1 CARTON 08/17/2015 1 50 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 08/17/2015 Labeler - LivCorp Inc. (248554748) Establishment Name Address ID/FEI Business Operations Les Emballages Facoteck Inc 245423439 manufacture(42903-008)
Trademark Results [LivRelief]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 LIVRELIEF 88061319 not registered Live/Pending |
Delivra Inc. 2018-08-01 |
 LIVRELIEF 77939612 4181213 Dead/Cancelled |
DELIVRA INC. 2010-02-19 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.