Warner Friends Sanitizer Clip and Clean, WE KNOW, Strawberry
Warner Friends Sanitizer Clip and Clean, WE KNOW, Strawberry by
Drug Labeling and Warnings
Warner Friends Sanitizer Clip and Clean, WE KNOW, Strawberry by is a Otc medication manufactured, distributed, or labeled by Mad Beauty USA LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
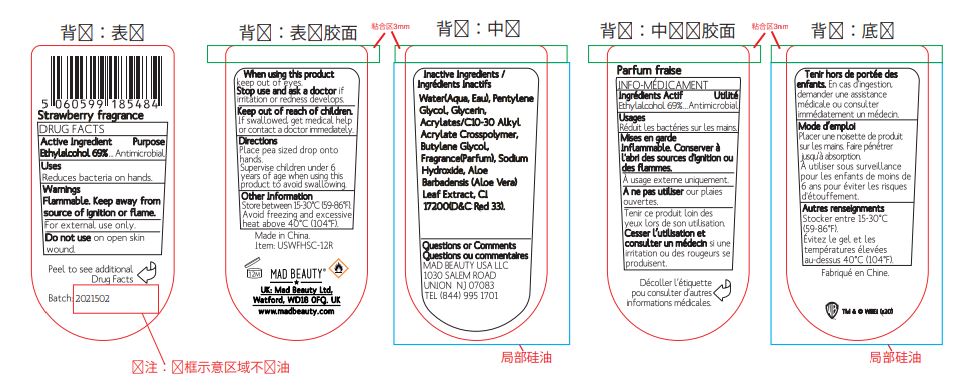
WARNER FRIENDS SANITIZER CLIP AND CLEAN, WE KNOW, STRAWBERRY- alcohol gel
Mad Beauty USA LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Warner Friends Sanitizer Clip and Clean, WE KNOW, Strawberry
Directions
- Place pea sized drop onto hands.
- Supervise children under 6 years of age when using this produt to avoid swallowing.
Other Information
Store between 15-30°C (59-86°F). Avoid freezing and excessive heat above 40°C (104°F).
| WARNER FRIENDS SANITIZER CLIP AND CLEAN, WE KNOW, STRAWBERRY
alcohol gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Mad Beauty USA LLC (117508758) |
Revised: 12/2021
Document Id: d2cc4d36-df8f-55e3-e053-2995a90a3019
Set id: aecf06f0-599c-6f08-e053-2995a90a3559
Version: 2
Effective Time: 20211210
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.