JACK BLACK INK BOOST TATTOO CARE by Jack Black L.L.C / Swiss American CDMO
JACK BLACK INK BOOST TATTOO CARE by
Drug Labeling and Warnings
JACK BLACK INK BOOST TATTOO CARE by is a Otc medication manufactured, distributed, or labeled by Jack Black L.L.C, Swiss American CDMO. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
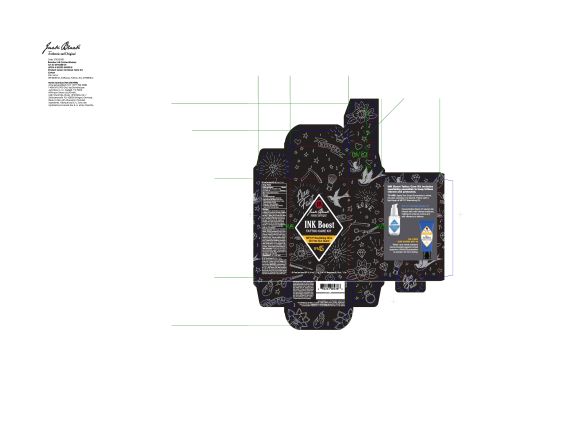
JACK BLACK INK BOOST TATTOO CARE- octinoxate, octisalate, zinc oxide lotion
Jack Black L.L.C
----------
Directions
- Apply liberally15 minutes before sun exporsure
- Reapply: After 80 minutes of swimming or sweating
- Immediately after towel drying
- At least every two hours
- Children under 6 months: Ask a doctor
- Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use sunscreen with a broad-spectrum SPF value of 15 or higher and othe sun protection measures including:
- Limit time in the sun, expecially from 10 a.m. - 2 p.m.
- Wear long-sleeved shirts, pants, hats, and sunglasses.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Inactive Ingredients
Water (Aqua), Cyclopentasiloxane, Ethylhexyl Isononanoate, Butylene Glycol, Lauryl PEG-9 Polydimethylsiloxyethyl Dimethicone, Calendula Officinalis (Calendula) Flower Extract*, Peucedanum Ostruthium Leaf Extract*, Buddleja Davidii Leaf Extract*, Artemisia Ubelliformis Extract*, Leontopodium Alpinum (Edelweiss) Extract*, Dimethicone, Dimethicone/Vinyl Dimethicone Crosspolymer, Phenoxyethanol, Sodium Chloride, Triethoxycaprylylsilane, Dimethicone/PEG-10/15 Crosspolymer, Iodopropynyl Butylcarbamate, Ascorbyl Palmitate, Retinyl Palmitate
*Certified Organic
| JACK BLACK INK BOOST TATTOO CARE
octinoxate, octisalate, zinc oxide lotion |
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Labeler - Jack Black L.L.C (847024036) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Swiss American CDMO | 080170933 | manufacture(66738-381) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
 Jack Black
Jack Black