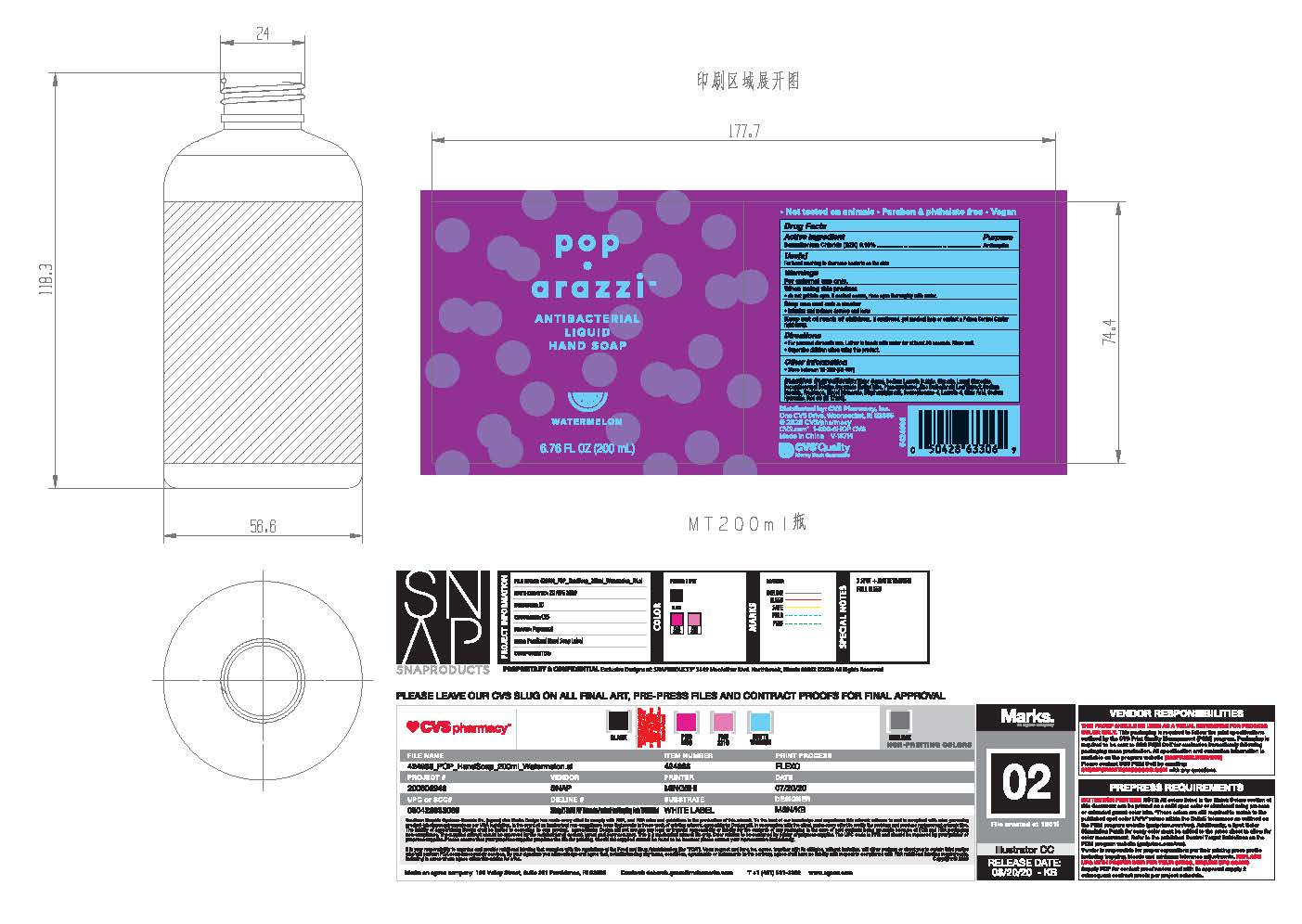
Pop•arazzi Watermelon Antibacterial Liquid Hand Soap
Pop Watermelon Antibacterial by
Drug Labeling and Warnings
Pop Watermelon Antibacterial by is a Otc medication manufactured, distributed, or labeled by MINGSHI TECHNOLOGY CO., LTD. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
POP WATERMELON ANTIBACTERIAL- benzalkonium chloride 0.13% liquid
MINGSHI TECHNOLOGY CO., LTD
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Poparazzi Watermelon Antibacterial Liquid Hand Soap
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away
Directions
For personal domestic use. Lather in hands with water for at least 30 seconds. Rinse well
Supervise children when using this product.
Inactive Ingredients
Water, Sodium Laureth Sulfate, Glycerin, Lauryl Glucoside, Cocoamidopropyl Betaine, Cocamide Methyl Mea, Phenoxyethanol, Aloe Barbadensis Leaf Extract, Sodium Chloride, Fragrance, Glycol Distearate, Ethylhexylglicerin, Benzophenone-4, Laureth-4, Citric Acid, Sodium Hydroxide, Red 33 (CI 17200)
| POP WATERMELON ANTIBACTERIAL
benzalkonium chloride 0.13% liquid |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - MINGSHI TECHNOLOGY CO., LTD (542992088) |