ANTIPHLAMINE by I World Pharmaceutical Co., Ltd.
ANTIPHLAMINE by
Drug Labeling and Warnings
ANTIPHLAMINE by is a Otc medication manufactured, distributed, or labeled by I World Pharmaceutical Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
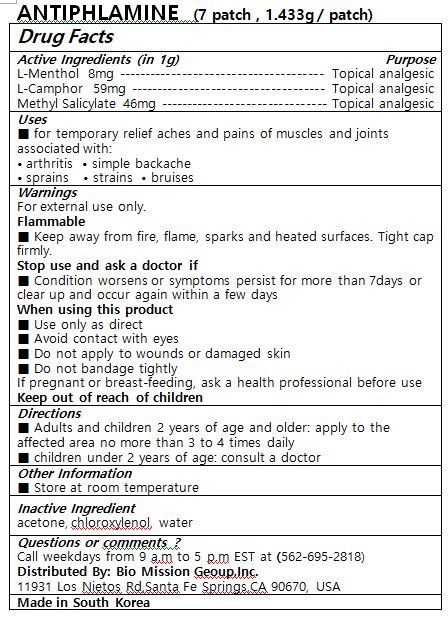
ANTIPHLAMINE- l-menthol, l-camphor, methyl salicylate patch
I World Pharmaceutical Co., Ltd.
----------
■ temporary relief aches and pains of muscles and joints associated with:
arthritis simple backache
sprains strains bruises
■ Adults and children 2 years of age and older: apply to the affected area no more than 3 to 4 times daily
■ children under 2 years of age: consult a doctor
Warnings
For external use only.
Flammable
■ Keep away from fire, flame, sparks and heated surfaces. Tight cap firmly.
Stop use and ask a doctor if
■ Condition worsens or symptoms persist for more than 7days or clear up and occur again within a few days
When using this product
■ Use only as direct
■ Avoid contact with eyes
■ Do not apply to wounds or damaged skin
■ Do not bandage tightly
If pregnant or breast-feeding, ask a health professional before use
Keep out of reach of children
| ANTIPHLAMINE
l-menthol, l-camphor, methyl salicylate patch |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - I World Pharmaceutical Co., Ltd. (688222857) |
| Registrant - I World Pharmaceutical Co., Ltd. (688222857) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| I-WORLD PHARM CO.,LTD. | 688222857 | manufacture(73442-0010) | |