Motion Sickness by Chain Drug Consortium, LLC Drug Facts
Motion Sickness by
Drug Labeling and Warnings
Motion Sickness by is a Otc medication manufactured, distributed, or labeled by Chain Drug Consortium, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
MOTION SICKNESS- dimenhydrinate tablet
Chain Drug Consortium, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
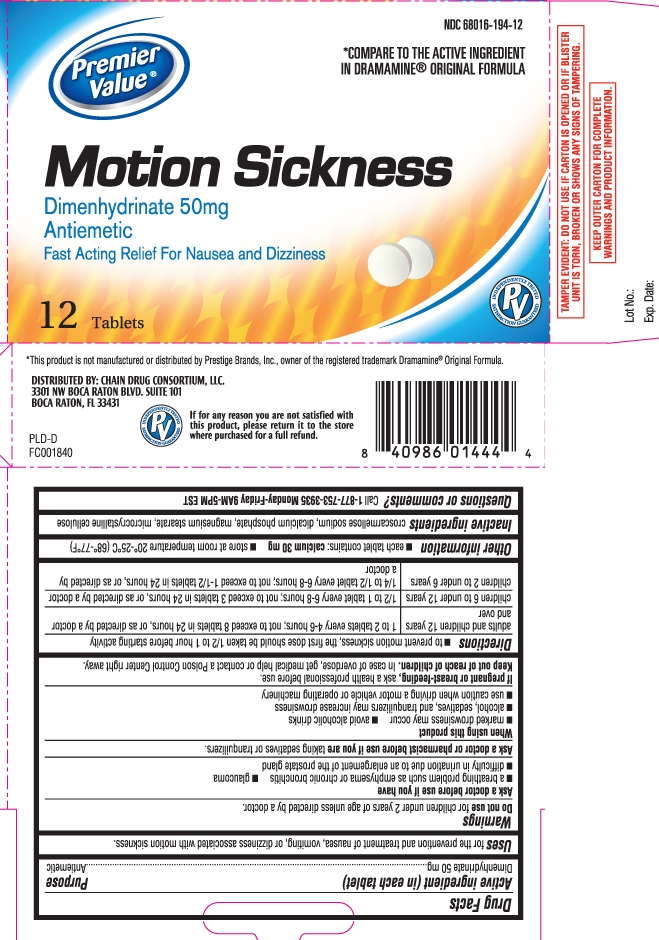
Drug Facts
Uses
for the prevention and treatment of nausea, vomiting, or dizziness associated with motion sickness.
Warnings
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to an enlargement of the prostate gland
Directions
- to prevent motion sickness, the first dose should be taken 1/2 to 1 hour before starting activity
| adults and children 12 years and over | 1 to 2 tablets every 4-6 hours; not to exceed 8 tablets in 24 hours, or as directed by a doctor |
| children 6 to under 12 years | 1/2 to 1 tablet every 6-8 hours; not to exceed 3 tablets in 24 hours, or as directed by a doctor |
| children 2 to under 6 years | 1/4 to 1/2 tablet every 6-8 hours; not to exceed 1-1/2 tablets in 24 hours, or as directed by a doctor |
Inactive ingredients
croscarmellose sodium, dicalcium phosphate, magnesium stearate, microcrystalline cellulose
Principal Display Panel
*COMPARE TO THE ACTIVE INGREDIENT IN DRAMAMINE® ORIGINAL FORMULA
Motion Sickness
Dimenhydrinate 50 mg
Antiemetic
Fast Acting Relief For Nausea and Dizziness
Tablets
*This product is not manufactured or distributed by Prestige Brands, Inc., owner of the registered trademark Dramamine® Original Formula.
DISTRIBUTED BY: CHAIN DRUG CONSORTIUM, LLC.
3301 NW BOCA RATON BLVD. SUITE 101
BOCA RATON, FL 33431
TAMPER EVIDENT: DO NOT USE IF CARTON IS OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
| MOTION SICKNESS
dimenhydrinate tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Chain Drug Consortium, LLC (101668460) |