CETACAINE ANESTHETIC- benzocaine, butamben, and tetracaine hydrochloride aerosol, spray
Cetacaine Anesthetic by
Drug Labeling and Warnings
Cetacaine Anesthetic by is a Prescription medication manufactured, distributed, or labeled by Cetylite Industries, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- DESCRIPTION
- SPL UNCLASSIFIED SECTION
- STORAGE AND HANDLING
-
Action
The onset of Cetacaine-produced anesthesia is rapid (approximately 30 seconds) and the duration of anesthesia is typically 30-60 minutes, when used as directed. This effect is due to the rapid onset, but short duration of action of Benzocaine coupled with the slow onset, but extended duration of Tetracaine HCl and bridged by the intermediate action of Butamben.
These agents act by reversibly blocking nerve conduction. Speed and duration of action is determined by the ability of the agent to be absorbed by the mucous membrane and nerve sheath and then to diffuse out, and ultimately be metabolized (primarily by plasma cholinesterases) to inert metabolites which are excreted in the urine.
-
Indications
Cetacaine is a topical anesthetic indicated for the production of anesthesia of all accessible mucous membrane except the eyes. Cetacaine Spray is indicated for use to control pain or gagging.
Cetacaine in all forms is indicated to control pain and for use for surgical or endoscopic or other procedures in the ear, nose, mouth, pharynx, larynx, trachea, bronchi, and esophagus. It may also be used for vaginal or rectal procedures when feasible.
-
Dosage and Administration
Cetacaine Spray should be applied for approximately one second or less for normal anesthesia. Only a limited quantity of Cetacaine is required for anesthesia. Spray in excess of two seconds is contraindicated. Each one-second spray contains an average of 200 mg of product, not including propellant.
To apply, insert the cannula firmly onto the protruding plastic stem on the bottle and press the cannula forward to actuate the spray valve. The cannula may be removed and reinserted as many times as required for cleaning, or sterilization, and is autoclavable.
Cetacaine Liquid
Apply 200 mg liquid (approximately 0.2 mL) directly to tissue. Liquid in excess of 400 mg (approximately 0.4 mL) is contraindicated.
To apply, remove and discard the shipping cap from the bottle. Replace it with the supplied Luer-lock cap. Caution: Do not over-tighten. Remove small cap from luer-lock port, retaining it for replacement after use. Port allows for a single dip of a cotton or brush applicator for application directly to accessible mucous membrane. For application into periodontal pockets, Cetylite Luer-lock syringes and Microcapillary Delivery Tips are recommended. Cetylite Syringes are clearly marked in (4) 0.1 mL increments. To fill, attach syringe to port by gently twisting clockwise until secure. Invert and draw desired amount of liquid into the syringe. Remove filled syringe and attach Microcapillary Delivery Tip to the syringe. Tip may be bent to improve access. Apply dropwise to accessible mucous membrane (such as buccal and lingual sulcus) by slowly depressing the syringe plunger. Discard all applicators after use.
An appropriate pediatric dosage has not been established for Cetacaine Spray or Cetacaine Liquid.
Dosages should be reduced in the debilitated elderly, acutely ill, and very young patients (i.e., children 2 years and older).
Do not use Cetacaine Spray or Cetacaine Liquid to treat infants or children younger than 2 years.
Tissue need not be dried prior to application of Cetacaine. Cetacaine should be applied directly to the site where pain control is required. Anesthesia is produced within one minute with an approximate duration of thirty minutes. Each 200 mg dose of Cetacaine (Spray or Liquid) contains 28 mg of benzocaine, 4 mg of butamben and 4 mg of tetracaine HCl.
-
WARNINGS AND PRECAUTIONS
Methemoglobinemia
Cases of methemoglobinemia have been reported in association with local anesthetic use. Although all patients are at risk for methemoglobinemia, patients with glucose-6-phosphate dehydrogenase deficiency, congenital or idiopathic methemoglobinemia, cardiac or pulmonary compromise, infants under 6 months of age, and concurrent exposure to oxidizing agents or their metabolites are more susceptible to developing clinical manifestations of the condition. If local anesthetics must be used in these patients, close monitoring for symptoms and signs of methemoglobinemia is recommended.
Signs and symptoms of methemoglobinemia may occur immediately or may be delayed some hours after exposure and are characterized by a cyanotic skin discoloration and abnormal coloration of the blood. Methemoglobin levels may continue to rise; therefore, immediate treatment is required to avert more serious central nervous system and cardiovascular adverse effects, including seizures, coma, arrhythmias, and death. Discontinue Cetacaine and any other oxidizing agents. Depending on the severity of the symptoms, patients may respond to supportive care, i.e., oxygen therapy, hydration. More severe symptoms may require treatment with methylene blue, exchange transfusion, or hyperbaric oxygen.
-
DRUG INTERACTIONS
Patients that are administered local anesthetics may be at increased risk of developing methemoglobinemia when concurrently exposed to the following oxidizing agents:
Class Examples Nitrates/Nitrites nitroglycerin, nitroprusside, nitric oxide, nitrous oxide Local anesthetics benzocaine, lidocaine, bupivacaine, mepivacaine, tetracaine, prilocaine, procaine, articaine, ropivacaine Antineoplastic agents cyclophosphamide, flutamide, rasburicase, ifosfamide, hydroxyurea Antibiotics dapsone, sulfonamides, nitrofurantoin, para-aminosalicylic acid Antimalarials chloroquine, primaquine Anticonvulsants phenytoin, sodium valproate, phenobarbital Other drugs acetaminophen, metoclopramide, sulfa drugs (i.e., sulfasalazine), quinine -
PATIENT COUNSELING INFORMATION
Inform patients that use of local anesthetics may cause methemoglobinemia, a serious condition that must be treated promptly. Advise patients or caregivers to stop use and seek immediate medical attention if they or someone in their care experience the following signs or symptoms: pale, gray, or blue colored skin (cyanosis); headache; rapid heart rate; shortness of breath; lightheadedness; or fatigue.
Hypersensitivity Reactions
Unpredictable adverse reactions (i.e. hypersensitivity, including anaphylaxis) are extremely rare.
Localized allergic reactions may occur after prolonged or repeated use of any aminobenzoate anesthetic. The most common adverse reaction caused by local anesthetics is contact dermatitis characterized by erythema and pruritus that may progress to vesiculation and oozing. This occurs most commonly in patients following prolonged self-medication, which is contraindicated. If rash, urticaria, edema, or other manifestations of allergy develop during use, the drug should be discontinued. To minimize the possibility of a serious allergic reaction, Cetacaine preparations should not be applied for prolonged periods except under continual supervision. Dehydration of the epithelium or an escharotic effect may also result from prolonged contact.
Use in Pregnancy
Safe use of Cetacaine has not been established with respect to possible adverse effects upon fetal development. Therefore, Cetacaine should not be used during early pregnancy, unless in the judgement of a physician, the potential benefits outweigh the unknown hazards. Routine precaution for the use of any topical anesthetic should be observed when Cetacaine is used.
-
Contraindications
Do not use Cetacaine Spray or Cetacaine Liquid to treat infants or children younger than 2 years.
Cetacaine is not suitable and should never be used for injection. Do not use on the eyes. To avoid excessive systemic absorption, Cetacaine should not be applied to large areas of denuded or inflamed tissue. Cetacaine should not be administered to patients who are hypersensitive to any of its ingredients or to patients known to have cholinesterase deficiencies. Tolerance may vary with the status of the patient.
Cetacaine should not be used under dentures or cotton rolls, as retention of the active ingredients under a denture or cotton roll could possibly cause an escharotic effect. Routine precaution for the use of any topical anesthetic should be observed when using Cetacaine.
- Autoclavable Cannula for Cetacaine Spray
-
Luer-lock Syringes and Microcapillary Delivery Tips for Cetacaine Liquid
- Cetacaine Liquid Syringes and Microcapillary Delivery Tips are supplied with the Cetacaine Liquid Chairside Kit (Item # 0218) and Clinical Kit (Item # 0212). They also can be purchased separately. Syringes are available in packs of 50 (Item # 0219S) or packs of 100 (Item # 0214). Delivery Tips are available in packs of 50 (Item # 0219T) or packs of 100 (Item # 0213).
-
How Supplied
- Cetacaine Spray, 20 g bottle, including propellant1 (Item # 0220, NDC: 10223-0201-3) which includes one cannula.
- Cetacaine Spray Single Patient Use, 5 g bottle, including propellant1 (Item # 0222, NDC: 10223-0201-4) which includes one cannula.
- Cetacaine Liquid Chairside Kit (Item # 0218 NDC: 10223-0202-6) which includes one 14 g bottle of Cetacaine Liquid with Luer-lock dispenser cap, 20 syringes and 20 delivery tips.
- Cetacaine Liquid Clinical Kit (Item # 0212, NDC: 10223-0202-5) which includes one 30 g bottle of Cetacaine Liquid with Luer-lock dispenser cap, 100 syringes and 100 delivery tips.
- Cetacaine Liquid, 14 g bottle (Item # 0203, NDC: 10223-0202-2) with Luer-lock dispenser cap.
- Cetacaine Liquid, 30 g bottle (Item # 0211, NDC: 10223-0202-4) with Luer-lock dispenser cap.
- 1 WARNING
Cetacaine Spray contains CFC-114 and CFC-11, substances which harm public health and environment by destroying ozone in the upper atmosphere. The propellant leak rate of Cetacaine Spray does not meet the USP requirements. - SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 20 g Bottle Box
NDC: 10223-0201-3
Item No. 0220Cetacaine®
Topical Anesthetic
SPRAYControl Pain
Control Gagging
Effective Only on
Mucous MembraneRx only
Exclusive Spray
Cannula Provides
Complete Control
(One Cannula Included)(Benzocaine 14.0%,
Butamben 2.0%, Tetracaine
Hydrochloride 2.0%)CETYLITE®
Cetylite Industries, Inc.
Pennsauken, NJ 08110-3293
www.cetylite.com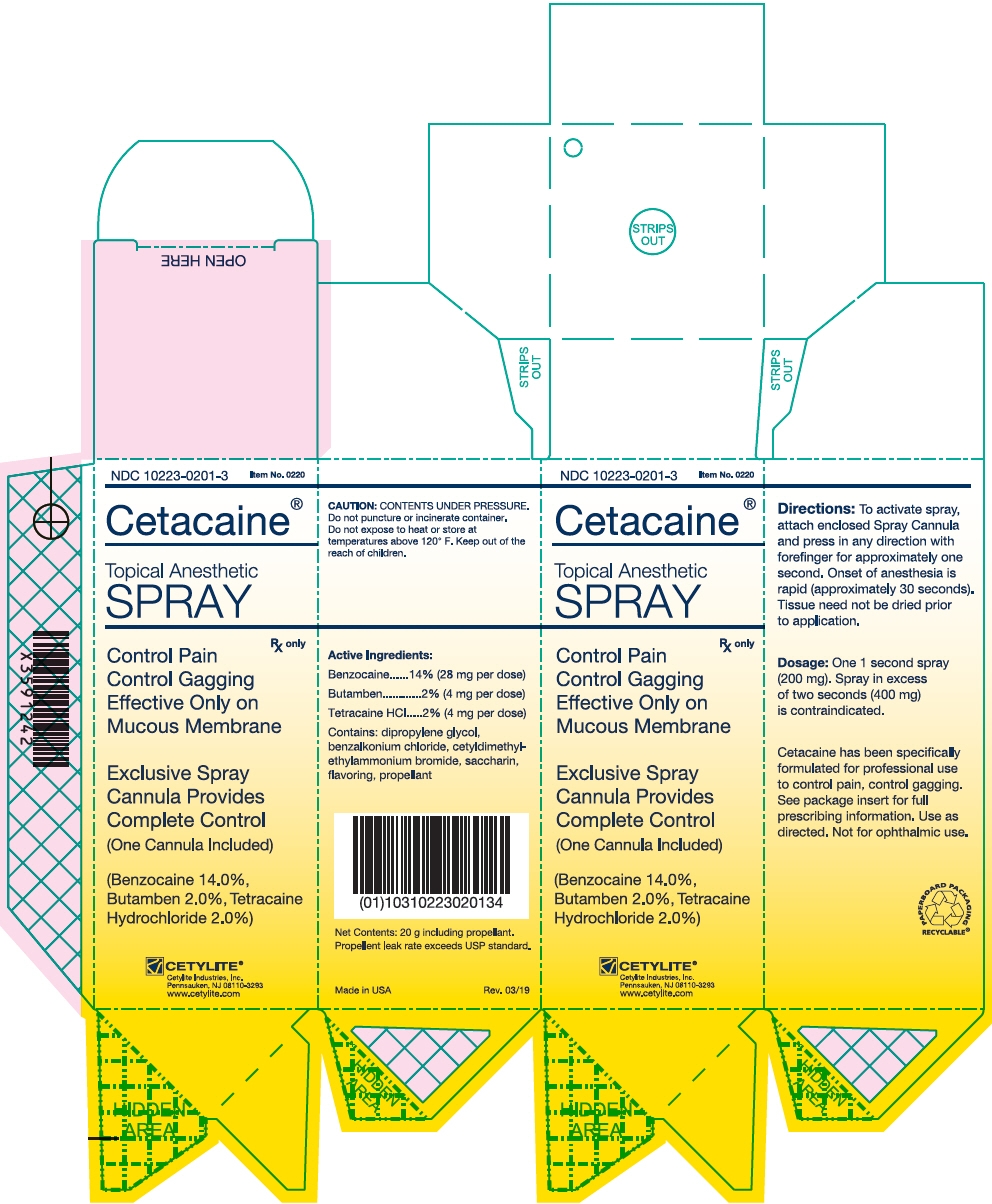
-
INGREDIENTS AND APPEARANCE
CETACAINE ANESTHETIC
benzocaine, butamben, and tetracaine hydrochloride aerosol, sprayProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 10223-0201 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Benzocaine (UNII: U3RSY48JW5) (Benzocaine - UNII:U3RSY48JW5) Benzocaine 0.028 g in 0.2 g Butamben (UNII: EFW857872Q) (Butamben - UNII:EFW857872Q) Butamben 0.004 g in 0.2 g Tetracaine Hydrochloride (UNII: 5NF5D4OPCI) (Tetracaine - UNII:0619F35CGV) Tetracaine Hydrochloride 0.004 g in 0.2 g Inactive Ingredients Ingredient Name Strength Dipropylene glycol (UNII: E107L85C40) Saccharin (UNII: FST467XS7D) Benzalkonium chloride (UNII: F5UM2KM3W7) Mecetronium bromide (UNII: 7ZNQ7FIO9K) Product Characteristics Color Score Shape Size Flavor BANANA Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 10223-0201-1 1 in 1 BOX 01/01/1958 01/31/2018 1 56 g in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 2 NDC: 10223-0201-3 1 in 1 BOX 01/01/2016 2 20 g in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 3 NDC: 10223-0201-4 1 in 1 BOX 11/01/2017 3 5 g in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 01/01/1958 Labeler - Cetylite Industries, Inc. (001283704) Establishment Name Address ID/FEI Business Operations Cetylite Industries, Inc. 001283704 MANUFACTURE(10223-0201) , ANALYSIS(10223-0201) , LABEL(10223-0201) , PACK(10223-0201)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.