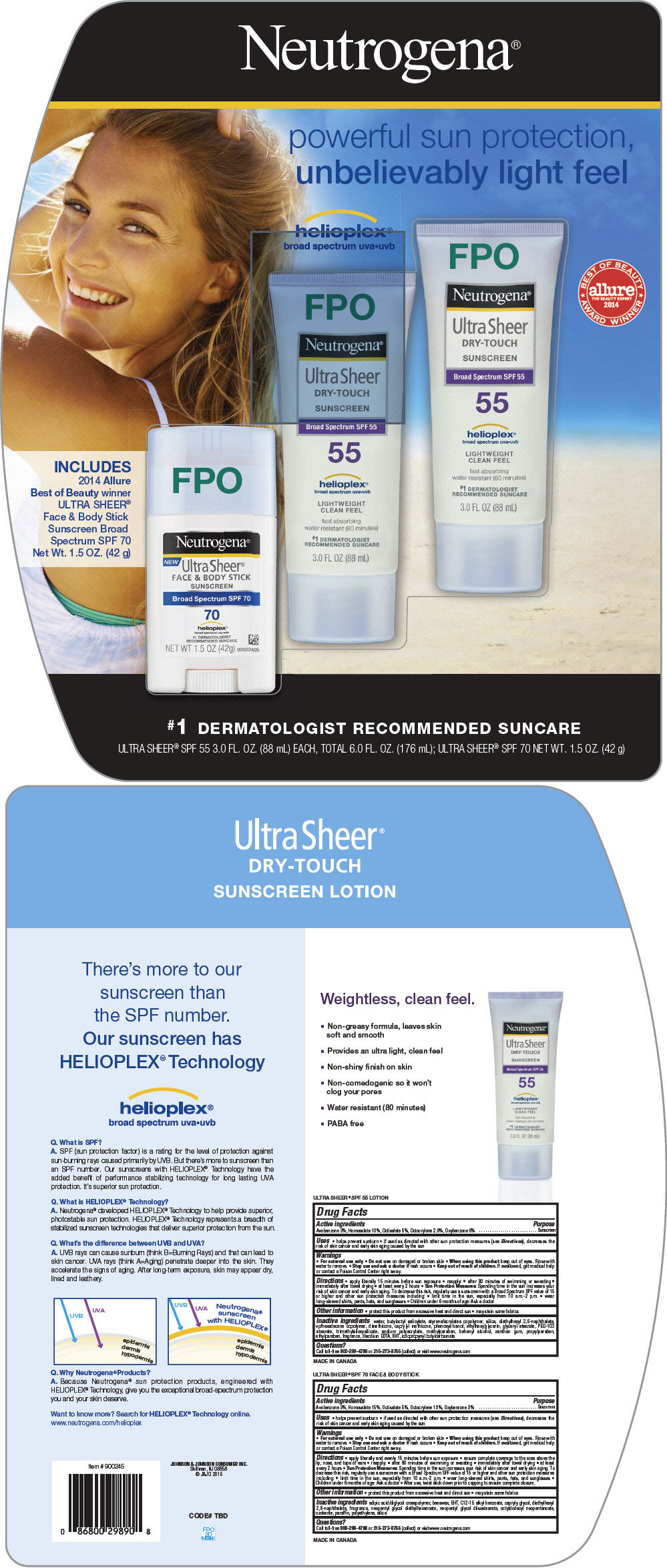
NEUTROGENA ULTRA SHEER SPF 55 AND SPF 70 SUNCARE KIT - avobenzone, homosalate, octisalate, octocrylene, oxybenzone
Johnson & Johnson Consumer Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Neutrogena® Ultra Sheer® DRY-TOUCH SUNSCREEN Broad Spectrum SPF 55
Drug Facts
Active ingredients
Avobenzone 3%
Homosalate 10%
Octisalate 5%
Octocrylene 2.8%
Oxybenzone 6%
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Warnings
-
Do not use on damaged or broken skin
-
When using this product keep out of eyes. Rinse with water to remove.
-
Stop use and ask a doctor if rash occurs
-
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- apply liberally 15 minutes before sun exposure
- reapply:
- after 80 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
-
Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- Children under 6 months of age: Ask a doctor
Other information
- protect this product from excessive heat and direct sun
- may stain some fabrics
Inactive ingredients
water, butyloctyl salicylate, styrene/acrylates copolymer, silica, diethylhexyl 2,6-naphthalate, VP/hexadecene copolymer, dimethicone, caprylyl methicone, phenoxyethanol, ethylhexylglycerin, glyceryl stearate, PEG-100 stearate, trimethylsiloxysilicate, sodium polyacrylate, methylparaben, behenyl alcohol, xanthan gum, propylparaben, ethylparaben, fragrance, disodium EDTA, BHT, iodopropynyl butylcarbamate
Questions?
Visit www.neutrogena.com or call toll-free 800-299-4786 or 215-273-8755 (collect)
Neutrogena Ultra Sheer Face & Body Stick Sunscreen Broad Spectrum SPF 70
Drug Facts
Active ingredients
Avobenzone 3%
Homosalate 15%
Octisalate 5%
Octocrylene 10%
Oxybenzone 3%
Use
- Helps prevent sunburn
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Warnings
For external use only
Do not use on damaged or broken skin
When using this product keep out of eyes. Rinse with water to remove
Stop use and ask a doctor if rash occurs
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- apply liberally and evenly 15 minutes before sun exposure
- ensure complete coverage to the area above the lip, nose, and tops of ears.
- reapply:
- 80 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum value of SPF of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m. – 2 p.m.
- Wear long-sleeve shirts, pants hats, and sunglasses
- Children under 6 months of age: Ask a doctor
Other information
- Protect this product from excessive heat and direct sun
- May stain some fabrics
Inactive Ingredients
Adipic Acid/Diglycol Crosspolymer, Beeswax, BHT, C12-15 Alkyl Benzoate, Caprylyl Glycol, Diethylhexyl 2,6-Naphthalate, Fragrance, Neopentyl Glycol Diethylhexanoate, Neopentyl Glycol Diisostearate, Octyldodecyl Neopentanoate, Ozokerite, Paraffin, Polyethylene, Silica
Questions or comments?
Call toll-free 800-299-4786 or 215-273-8755 (collect) or visit www.neutrogena.com
PRINCIPAL DISPLAY PANEL - Kit Carton
Neutrogena®
powerful sun protection,
unbelievably light feel
helioplex®
broad spectrum uvauvb
BEST OF BEAUTY
allure
THE BEAUTY EXPERT
2014
AWARD WINNER
INCLUDES
2014 Allure
Best of Beauty winner
ULTRA SHEER®
Face & Body Stick
Sunscreen Broad
Spectrum SPF 70
Net Wt. 1.5 OZ. (42 g)
#1 DERMATOLOGIST RECOMMENDED SUNCARE
ULTRA SHEER® SPF 55 3.0 FL. OZ. (88 mL) EACH, TOTAL 6.0 FL.OZ.(176 mL); ULTRA SHEER® SPF 70 NET WT.1.5 OZ. (42 g)