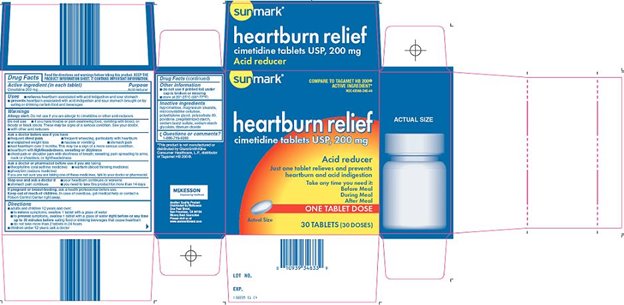
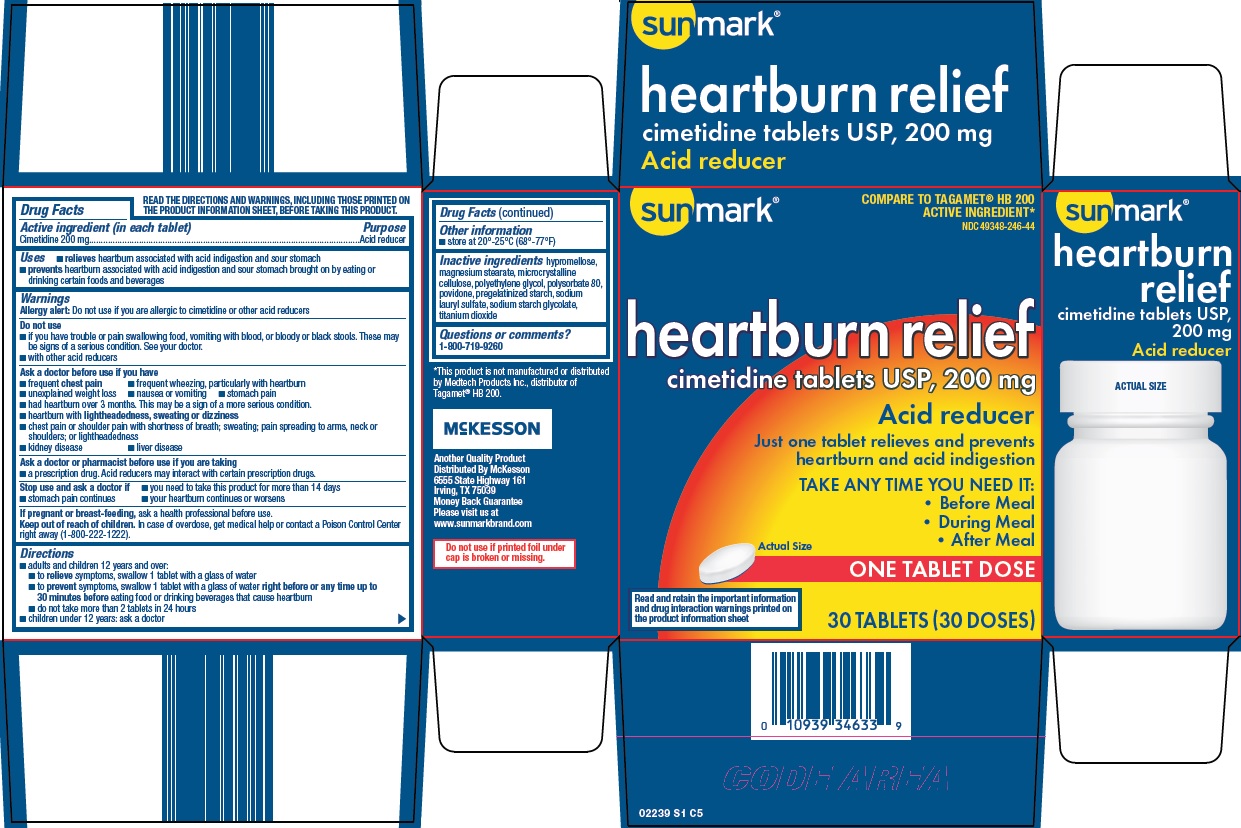
SUNMARK HEARTBURN RELIEF ACID REDUCER- cimetidine tablet, film coated
SunMark heartburn relief by
Drug Labeling and Warnings
SunMark heartburn relief by is a Otc medication manufactured, distributed, or labeled by Strategic Sourcing Services LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Allergy alert: Do not use if you are allergic to cimetidine or other acid reducers
Do not use
- if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition. See your doctor.
- with other acid reducers
Ask a doctor before use if you have
- frequent chest pain
- frequent wheezing, particularly with heartburn
- unexplained weight loss
- nausea or vomiting
- stomach pain
- had heartburn over 3 months. This may be a sign of a more serious condition.
- heartburn with lightheadedness, sweating or dizziness
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- kidney disease
- liver disease
Ask a doctor or pharmacist before use if you are
- taking
- a prescription drug. Acid reducers may interact with certain prescription drugs.
-
Directions
- adults and children 12 years and over:
- to relieve symptoms, swallow 1 tablet with a glass of water
- to prevent symptoms, swallow 1 tablet with a glass of water right before or any time up to 30 minutes before eating food or drinking beverages that cause heartburn
- do not take more than 2 tablets in 24 hours
- children under 12 years: ask a doctor
- Other information
- Inactive ingredients
- Questions or comments?
-
Consumer Information
Cimetidine Tablets, 200 mg
Acid Reducer
For Heartburn
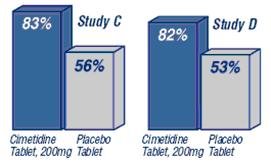
In clinical studies, Cimetidine Tablets, 200 mg was significantly better than placebo tablets in relieving and preventing heartburn symptoms.
Percent of Heartburn Episodes Relieved
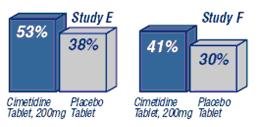
Percent of Patients with Prevention or Reduction of Heartburn Symptoms When Taken 30 Minutes Before a Meal
Percent of Patients with Prevention or Reduction of Heartburn Symptoms When Taken at Time of Meal
Drug Interaction Warnings:
Cimetidine Tablets, 200 mg affects some prescription medicines causing slightly higher levels of those medicines in the blood. Higher blood levels could lead to side effects in rare situations. If you currently take Theophylline (oral asthma medicine), Warfarin (blood thinning medicine) or Phenytoin (seizure medicine), consult your doctor before taking this product.
Brand names of some medicines which contain one of these ingredients include:
THEOPHYLLINE
THEO-DUR® Theochron® Slo-Bid® Uniphyl® Theo-24®
WARFARIN
Coumadin®
PHENYTOIN
Dilantin®
There may be other medicines that contain one of these ingredients. If in doubt about this or about possible effects of Cimetidine Tablets, 200 mg on any other medicines you are taking, talk to your doctor or pharmacist.
Tips for Managing Heartburn
- Do not lie flat or bend over soon after eating
- Do not eat late at night, or just before bedtime
- Certain foods or drinks are more likely to cause heartburn, such as rich, spicy, fatty, and fried foods, chocolate, caffeine, alcohol, even some fruits and vegetables
- Eat slowly and do not eat big meals
- If you are overweight, lose weight
- If you smoke, quit smoking
- Raise the head of your bed
- Wear loose fitting clothing around your stomach
Read the directions and warnings before taking this medication.
Questions or Comments?
1-800-719-9260
Distributed By
Perrigo®
Allegan, MI 49010
Rev 08/19
02200 00 J5
-
Principal Display Panel
COMPARE TO TAGAMET® HB 200 ACTIVE INGREDIENT
heartburn relief cimetidine tablets USP, 200 mg
Acid Reducer
Just one tablet relieves and prevents heartburn and acid indigestion
TAKE ANY TIME YOU NEED IT:
Before Meal
During Meal
After Meal
Actual Size
ONE TABLET DOSE
Read and retain the important information and drug interaction warnings printed on the product information sheet
30 TABLETS (30 DOSES)
-
INGREDIENTS AND APPEARANCE
SUNMARK HEARTBURN RELIEF ACID REDUCER
cimetidine tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 49348-246 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CIMETIDINE (UNII: 80061L1WGD) (CIMETIDINE - UNII:80061L1WGD) CIMETIDINE 200 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POVIDONE (UNII: FZ989GH94E) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE Score no score Shape OVAL Size 13mm Flavor Imprint Code L022 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49348-246-44 1 in 1 CARTON 09/19/2003 1 30 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA075285 09/19/2003 Labeler - Strategic Sourcing Services LLC (116956644)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.