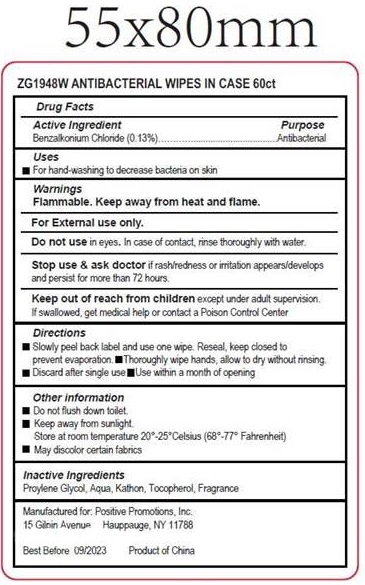
Antibacterial Wipes by Positive Promotions Inc. Antibacterial Wipes
Antibacterial Wipes by
Drug Labeling and Warnings
Antibacterial Wipes by is a Otc medication manufactured, distributed, or labeled by Positive Promotions Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ANTIBACTERIAL WIPES- benzalkonium chloride cloth
Positive Promotions Inc.
----------
Antibacterial Wipes
Warnings
Flammable. Keep away from heat and flame.For External use only.
Directions
- Slowly peel back label and use one wipe. Reseal, keep closed to prevent evaporation.
- Thoroughly wipe hands, allow to dry without rinsing.
- Discard after single use
- Use within a month of opening
| ANTIBACTERIAL WIPES
benzalkonium chloride cloth |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Positive Promotions Inc. (002401719) |
Revised: 10/2023
Document Id: 08bf1a51-d8de-edb2-e063-6294a90aedd4
Set id: af489317-86eb-9921-e053-2a95a90a4e6e
Version: 4
Effective Time: 20231027