769 Sensi-Care Clear Zinc
Sensi-Care by
Drug Labeling and Warnings
Sensi-Care by is a Otc medication manufactured, distributed, or labeled by Medline Industries, LP. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SENSI-CARE- dimethicon, zinc oxide cream
Medline Industries, LP
----------
769 Sensi-Care Clear Zinc
Uses
- helps treat and prevent diaper rash
- protects minor skin irritation associated with diaper rash and helps seal out wetness
Warnings
For external use only.
Directions
- change wet and soiled diapers promptly, cleanse the diaper area, and allow to dry
- apply product liberally as often as necessary, with each diaper change, especially at bedtime or anytime when exposure to wet diapers may be prolonged
Other information
- store at 15°C-30°C (59°F-86°F)
- *for infacts/neonates, please use under medical supervision
Inactive ingredients
butylene/ethylene/styrene copolymer, calamine, carthamus tinctorius (safflower) oleosomes, cellulose gum, cocos nucifera (coconut) oil, ethylene/propylene/styrene copolymer, gelatin, gossypium herbaceum (cotton) seed oil, hydrolyzed collagen, lanolin, microcrystalline wax, mineral oil, oleic acid, pectin, phenoxyethanol, punica granatum sterols, simmondsia chinensis (jojoba) seed oil, sodium hyaluronate, steric acid, theobroma cacao (cocoa) seed butter, tocopheryl acetate, water
| SENSI-CARE
dimethicon, zinc oxide cream |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Medline Industries, LP (025460908) |
| Registrant - Medline Industries, LP (025460908) |
Revised: 7/2024
Document Id: 1c5c3c75-0857-dbde-e063-6394a90a6ecc
Set id: af5fd83e-7eaa-3a02-e053-2a95a90a3a9d
Version: 5
Effective Time: 20240703
Trademark Results [Sensi-Care]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SENSI-CARE 98606858 not registered Live/Pending |
Keyflow (UK) Ltd 2024-06-18 |
 SENSI-CARE 86803611 5448912 Live/Registered |
ConvaTec Inc. 2015-10-29 |
 SENSI-CARE 86123818 4672858 Live/Registered |
The Procter & Gamble Company 2013-11-20 |
 SENSI-CARE 75700756 2618533 Live/Registered |
CONVATEC INC. 1999-05-07 |
 SENSI-CARE 75700755 not registered Dead/Abandoned |
E.R. SQUIBB & SONS, INC. 1999-05-07 |
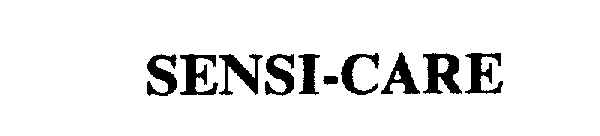 SENSI-CARE 75371562 2322222 Live/Registered |
ConvaTec Inc. 1997-10-10 |
 SENSI-CARE 73551504 1387137 Dead/Cancelled |
SOUTHERN PLAINS MEDICAL CENTER, INC. 1985-08-02 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
