GRANISETRON HYDROCHLORIDE injection
GRANISETRON HYDROCHLORIDE by
Drug Labeling and Warnings
GRANISETRON HYDROCHLORIDE by is a Prescription medication manufactured, distributed, or labeled by WOCKHARDT LIMITED. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use granisetron hydrochloride injection safely and effectively. See full prescribing information for granisetron hydrochloride injection.
Granisetron Hydrochloride Injection, USP, for intravenous use
Initial U.S. Approval: 1993INDICATIONS AND USAGE
Granisetron hydrochloride injection, USP is a serotonin-3 (5-HT3) receptor antagonist indicated for:
- Prevention of nausea and/or vomiting associated with initial and repeat courses of emetogenic cancer therapy, including high-dose cisplatin. (1)
DOSAGE AND ADMINISTRATION
Prevention of chemotherapy-induced nausea and vomiting (2.1):- Recommended dosage is 10 mcg/kg intravenously within 30 minutes before initiation of chemotherapy
- Pediatric patients (2 to 16 years): Recommended dosage is 10 mcg/kg
DOSAGE FORMS AND STRENGTHS
- Injection 1 mg/mL (free base). (3)
CONTRAINDICATIONS
- Hypersensitivity to granisetron or to any of its components. (4)
WARNINGS AND PRECAUTIONS
- Granisetron hydrochloride injection does not stimulate gastric or intestinal peristalsis and should not be used instead of nasogastric suction. (5.1)
- QT prolongation has been reported with granisetron hydrochloride injection. Use with caution in patients with pre-existing arrhythmias or cardiac conduction disorders. (5.2)
- Hypersensitivity reactions, such as anaphylaxis, shortness of breath, hypotension, and urticaria, may occur in patients with known hypersensitivity to other selective 5-HT3 receptor antagonists. (5.3)
ADVERSE REACTIONS
Most common adverse reactions:
- Chemotherapy-induced nausea and vomiting (≥3%): Headache, and constipation (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Wockhardt USA LLC. at 1-800-346-6854 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Granisetron hydrochloride injection has been administered safely with benzodiazepines, neuroleptics, and anti-ulcer medications. (7)
- Does not appear to interact with emetogenic cancer chemotherapies. (7)
- Inducers or inhibitors of CYP450 enzymes may change the clearance and therefore the half-life of granisetron. (7)
- Coadministration of granisetron hydrochloride injection with drugs known to prolong the QT interval and/or are arrhythmogenic may result in clinical consequences. (7)
USE IN SPECIFIC POPULATIONS
- Pregnancy: Use only if clearly needed. (8.1)
- Nursing mothers: Caution should be exercised when administered to a nursing woman. (8.3)
- Geriatric use: No differences in responses between the elderly and younger patients were observed in reported clinical experience. (8.5)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 9/2011
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Prevention of Chemotherapy-Induced Nausea and Vomiting
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Gastric or Intestinal Peristalsis
5.2 Cardiovascular Events
5.3 Hypersensitivity Reactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Chemotherapy-Induced Nausea and Vomiting
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Prevention of Chemotherapy-Induced Nausea and Vomiting
Adult Patients
The recommended dosage for granisetron hydrochloride injection is 10 mcg/kg administered intravenously within 30 minutes before initiation of chemotherapy, and only on the day(s) chemotherapy is given.
Infusion Preparation
Granisetron hydrochloride injection may be administered intravenously either undiluted over 30 seconds, or diluted with 0.9% Sodium Chloride or 5% Dextrose and infused over 5 minutes.
Stability
Intravenous infusion of granisetron hydrochloride injection should be prepared at the time of administration. However, granisetron hydrochloride injection has been shown to be stable for at least 24 hours when diluted in 0.9% Sodium Chloride or 5% Dextrose and stored at room temperature under normal lighting conditions.
As a general precaution, granisetron hydrochloride injection should not be mixed in solution with other drugs. Parenteral drug products should be inspected visually for particulate matter and discoloration before administration whenever solution and container permit.
Pediatric Patients
The recommended dose in pediatric patients 2 to 16 years of age is 10 mcg/kg [see Clinical Studies (14)]. Pediatric patients under 2 years of age have not been studied.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Gastric or Intestinal Peristalsis
Granisetron hydrochloride injection is not a drug that stimulates gastric or intestinal peristalsis. It should not be used instead of nasogastric suction. The use of granisetron hydrochloride injection in patients following abdominal surgery or in patients with chemotherapy-induced nausea and vomiting may mask a progressive ileus and/or gastric distention.5.2 Cardiovascular Events
An adequate QT assessment has not been conducted, but QT prolongation has been reported with granisetron hydrochloride injection. Therefore, granisetron hydrochloride injection should be used with caution in patients with pre-existing arrhythmias or cardiac conduction disorders, as this might lead to clinical consequences. Patients with cardiac disease, on cardio-toxic chemotherapy, with concomitant electrolyte abnormalities and/or on concomitant medications that prolong the QT interval are particularly at risk.
-
6 ADVERSE REACTIONS
QT prolongation has been reported with granisetron hydrochloride injection [see Warnings and Precautions (5.2) and Drug Interactions (7)].6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in patients.
Chemotherapy-Induced Nausea and Vomiting
The following have been reported during controlled clinical trials or in the routine management of patients. The percentage figures are based on clinical trial experience only. Table 1 gives the comparative frequencies of the two most commonly reported adverse reactions (≥3%) in patients receiving granisetron hydrochloride injection, in single-day chemotherapy trials. These patients received chemotherapy, primarily cisplatin, and intravenous fluids during the 24-hour period following granisetron hydrochloride injection administration. Reactions were generally recorded over seven days post-granisetron hydrochloride injection administration.
1Metoclopramide/dexamethasone and phenothiazines/ dexamethasone.Table 1 Principal Adverse Reactions in Clinical Trials-Single-Day Chemotherapy Percent of Patients With Reaction Granisetron Hydrochloride Injection
40 mcg/kg
(n=1268)Comparator1
(n=422)Headache 14% 6% Constipation 3% 3%
Additional adverse events reported in clinical trials were asthenia, somnolence and diarrhea.
In over 3,000 patients receiving granisetron hydrochloride injection (2 to 160 mcg/kg) in single-day and multiple-day clinical trials with emetogenic cancer therapies, adverse events, other than those adverse reactions listed in Table 1, were observed; attribution of many of these events to granisetron hydrochloride injection is uncertain.
Hepatic: In comparative trials, mainly with cisplatin regimens, elevations of AST and ALT (>2 times the upper limit of normal) following administration of granisetron hydrochloride injection occurred in 2.8% and 3.3% of patients, respectively. These frequencies were not significantly different from those seen with comparators (AST: 2.1%; ALT: 2.4%).
Cardiovascular: Hypertension (2%); hypotension, arrhythmias such as sinus bradycardia, atrial fibrillation, varying degrees of A-V block, ventricular ectopy including non-sustained tachycardia, and ECG abnormalities have been observed rarely.
Central Nervous System: Agitation, anxiety, CNS stimulation and insomnia were seen in less than 2% of patients. Extrapyramidal syndrome occurred rarely and only in the presence of other drugs associated with this syndrome.
Hypersensitivity: Rare cases of hypersensitivity reactions, sometimes severe (eg, anaphylaxis, shortness of breath, hypotension, urticaria) have been reported.
Other: Fever (3%), taste disorder (2%), skin rashes (1%). In multiple-day comparative studies, fever occurred more frequently with granisetron hydrochloride injection (8.6%) than with comparative drugs (3.4%, P<0.014), which usually included dexamethasone.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of granisetron hydrochloride injection. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to granisetron hydrochloride injection exposure.
QT prolongation has been reported with granisetron hydrochloride injection [see Warnings and Precautions (5.2) and Drug Interactions (7)]. -
7 DRUG INTERACTIONS
Granisetron does not induce or inhibit the cytochrome P-450 drug-metabolizing enzyme system in vitro. There have been no definitive drug-drug interaction studies to examine pharmacokinetic or pharmacodynamic interaction with other drugs; however, in humans, granisetron hydrochloride injection has been safely administered with drugs representing benzodiazepines, neuroleptics and anti-ulcer medications commonly prescribed with antiemetic treatments. Granisetron hydrochloride injection also does not appear to interact with emetogenic cancer chemotherapies. Because granisetron is metabolized by hepatic cytochrome P-450 drug-metabolizing enzymes, inducers or inhibitors of these enzymes may change the clearance and, hence, the half-life of granisetron. No specific interaction studies have been conducted in anesthetized patients. In addition, the activity of the cytochrome P-450 subfamily 3A4 (involved in the metabolism of some of the main narcotic analgesic agents) is not modified by granisetron hydrochloride injection in vitro.
In in vitro human microsomal studies, ketoconazole inhibited ring oxidation of granisetron hydrochloride injection. However, the clinical significance of in vivo pharmacokinetic interactions with ketoconazole is not known. In a human pharmacokinetic study, hepatic enzyme induction with phenobarbital resulted in a 25% increase in total plasma clearance of intravenous granisetron hydrochloride injection. The clinical significance of this change is not known.
QT prolongation has been reported with granisetron hydrochloride injection. Use of granisetron hydrochloride injection in patients concurrently treated with drugs known to prolong the QT interval and/or are arrhythmogenic may result in clinical consequences. -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B
Reproduction studies have been performed in pregnant rats at intravenous doses up to 9 mg/kg/day (54 mg/m2/day, 146 times the recommended human dose based on body surface area) and pregnant rabbits at intravenous doses up to 3 mg/kg/day (35.4 mg/m2/day, 96 times the recommended human dose based on body surface area) and have revealed no evidence of impaired fertility or harm to the fetus due to granisetron. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.8.3 Nursing Mothers
It is not known whether granisetron is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when granisetron hydrochloride injection is administered to a nursing woman.
- 10 OVERDOSAGE
-
11 DESCRIPTION
Granisetron hydrochloride injection, USP is a serotonin-3 (5-HT3) receptor antagonist. Chemically it is endo-N-(9-methyl-9-azabicyclo [3.3.1] non-3-yl)-1-methyl-1H-indazole-3-carboxamide hydrochloride with a molecular weight of 348.9 (312.4 free base). Its empirical formula is C18H24N4OHcl, while its chemical structure is:
Granisetron hydrochloride is a white crystalline powder, freely soluble in water and normal saline at 20°C. Granisetron hydrochloride injection, USP is a clear, colorless, sterile, nonpyrogenic, aqueous solution for intravenous administration.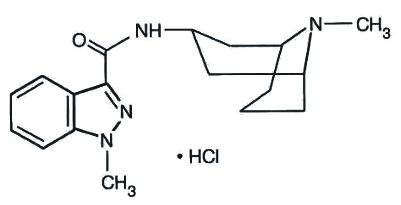
Granisetron hydrochloride injection, USP 1 mg/mL is available in a 4 mL multi-use vial.
1 mg/ mL: Each mL contains 1.12 mg granisetron hydrochloride equivalent to granisetron, 1 mg; sodium chloride, 9 mg; citric acid, 2 mg; methylparaben, 1.8 mg; propylparaben, 0.2 mg; as a preservative and sodium hydroxide and hydrochloric acid (used to adjust pH). The solution's pH ranges from 4.0 to 6.0. -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Granisetron is a selective 5-hydroxytryptamine3 (5-HT3) receptor antagonist with little or no affinity for other serotonin receptors, including 5-HT1; 5-HT1A; 5-HT1B/C; 5-HT2; for alpha1-, alpha2- or beta-adrenoreceptors; for dopamine-D2; or for histamine-H1; benzodiazepine; picrotoxin or opioid receptors.
Serotonin receptors of the 5-HT3 type are located peripherally on vagal nerve terminals and centrally in the chemoreceptor trigger zone of the area postrema. During chemotherapy-induced vomiting, mucosal enterochromaffin cells release serotonin, which stimulates 5-HT3 receptors. This evokes vagal afferent discharge and may induce vomiting. Animal studies demonstrate that, in binding to 5-HT3 receptors, Granisetron blocks serotonin stimulation and subsequent vomiting after emetogenic stimuli such as cisplatin. In the ferret animal model, a single granisetron injection prevented vomiting due to high-dose cisplatin or arrested vomiting within 5 to 30 seconds.
In most human studies, granisetron has had little effect on blood pressure, heart rate or ECG. No evidence of an effect on plasma prolactin or aldosterone concentrations has been found in other studies.
Granisetron hydrochloride injection exhibited no effect on oro-cecal transit time in normal volunteers given a single intravenous infusion of 50 mcg/kg or 200 mcg/kg. Single and multiple oral doses slowed colonic transit in normal volunteers.12.3 Pharmacokinetics
Chemotherapy-Induced Nausea and Vomiting
In adult cancer patients undergoing chemotherapy and in volunteers, mean pharmacokinetic data obtained from an infusion of a single 40 mcg/kg dose of granisetron hydrochloride injection are shown in Table 2.
*5-minute infusion.Table 2 Pharmacokinetic Parameters in Adult Cancer Patients Undergoing Chemotherapy and in Volunteers, Following a Single Intravenous 40 mcg/kg Dose of Granisetron Hydrochloride Injection Peak Plasma Concentration
(ng/mL)Terminal Phase Plasma Half-Life
(h)Total Clearance
(L/h/kg)Volume of Distribution
(L/kg)Cancer Patients Mean 63.8* 8.95* 0.38* 3.07* Range 18.0 to 176 0.90 to 31.1 0.14 to 1.54 0.85 to 10.4 Volunteers 21 to 42 years Mean 64.3† 4.91† 0.79† 3.04† Range 11.2 to 182 0.88 to 15.2 0.20 to 2.56 1.68 to 6.13 65 to 81 years Mean 57.0† 7.69† 0.44† 3.97† Range 14.6 to 153 2.65 to 17.7 0.17 to 1.06 1.75 to 7.01
†3- minute infusion.
Distribution
Plasma protein binding is approximately 65% and granisetron distributes freely between plasma and red blood cells.
Metabolism
Granisetron metabolism involves N-demethylation and aromatic ring oxidation followed by conjugation. In vitro liver microsomal studies show that granisetron's major route of metabolism is inhibited by ketoconazole, suggestive of metabolism mediated by the cytochrome P-450 3A subfamily. Animal studies suggest that some of the metabolites may also have 5-HT3 receptor antagonist activity.
Elimination
Clearance is predominantly by hepatic metabolism. In normal volunteers, approximately 12% of the administered dose is eliminated unchanged in the urine in 48 hours. The remainder of the dose is excreted as metabolites, 49% in the urine, and 34% in the feces.
Subpopulations
Gender
There was high inter- and intra-subject variability noted in these studies. No difference in mean AUC was found between males and females, although males had a higher Cmax generally.
Elderly
The ranges of the pharmacokinetic parameters in elderly volunteers (mean age 71 years), given a single 40 mcg/kg intravenous dose of granisetron hydrochloride injection, were generally similar to those in younger healthy volunteers; mean values were lower for clearance and longer for half-life in the elderly patients (see Table 2).
Pediatric Patients
A pharmacokinetic study in pediatric cancer patients (2 to 16 years of age), given a single 40 mcg/kg intravenous dose of granisetron hydrochloride injection, showed that volume of distribution and total clearance increased with age. No relationship with age was observed for peak plasma concentration or terminal phase plasma half-life. When volume of distribution and total clearance are adjusted for body weight, the pharmacokinetics of granisetron are similar in pediatric and adult cancer patients.
Renal Failure Patients
Total clearance of granisetron was not affected in patients with severe renal failure who received a single 40 mcg/kg intravenous dose of granisetron hydrochloride injection.
Hepatically Impaired Patients
A pharmacokinetic study in patients with hepatic impairment due to neoplastic liver involvement showed that total clearance was approximately halved compared to patients without hepatic impairment. Given the wide variability in pharmacokinetic parameters noted in patients, dosage adjustment in patients with hepatic functional impairment is not necessary.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 24-month carcinogenicity study, rats were treated orally with granisetron 1, 5 or 50 mg/kg/day (6, 30 or 300 mg/m2/day). The 50 mg/kg/day dose was reduced to 25 mg/kg/day (150 mg/m2/day) during week 59 due to toxicity. For a 50 kg person of average height (1.46 m2 body surface area), these doses represent 16, 81 and 405 times the recommended clinical dose (0.37 mg/m2, iv) on a body surface area basis. There was a statistically significant increase in the incidence of hepatocellular carcinomas and adenomas in males treated with 5 mg/kg/day (30 mg/m2/day, 81 times the recommended human dose based on body surface area) and above, and in females treated with 25 mg/kg/day (150 mg/m2/day, 405 times the recommended human dose based on body surface area). No increase in liver tumors was observed at a dose of 1 mg/kg/day (6 mg/m2/day, 16 times the recommended human dose based on body surface area) in males and 5 mg/kg/day (30 mg/m2/day, 81 times the recommended human dose based on body surface area) in females. In a 12-month oral toxicity study, treatment with granisetron 100 mg/kg/day (600 mg/m2/day, 1622 times the recommended human dose based on body surface area) produced hepatocellular adenomas in male and female rats while no such tumors were found in the control rats. A 24-month mouse carcinogenicity study of granisetron did not show a statistically significant increase in tumor incidence, but the study was not conclusive.
Because of the tumor findings in rat studies, granisetron hydrochloride injection should be prescribed only at the dose and for the indication recommended [see Indications and Usage (1) and Dosage and Administration (2)].
Granisetron was not mutagenic in an in vitro Ames test and mouse lymphoma cell forward mutation assay, and in vivo mouse micronucleus test and in vitro and ex vivo rat hepatocyte UDS assays. It, however, produced a significant increase in UDS in HeLa cells in vitro and a significant increased incidence of cells with polyploidy in an in vitro human lymphocyte chromosomal aberration test.
Granisetron at subcutaneous doses up to 6 mg/kg/day (36 mg/m2/day, 97 times the recommended human dose based on body surface area) was found to have no effect on fertility and reproductive performance of male and female rats.
-
14 CLINICAL STUDIES
14.1 Chemotherapy-Induced Nausea and Vomiting
Single-Day Chemotherapy
Cisplatin-Based Chemotherapy
In a double-blind, placebo-controlled study in 28 cancer patients, granisetron hydrochloride injection, administered as a single intravenous infusion of 40 mcg/kg, was significantly more effective than placebo in preventing nausea and vomiting induced by cisplatin chemotherapy (see Table 3).
1Cisplatin administration began within 10 minutes of granisetron hydrochloride injection infusion and continued for 1.5 to 3.0 hours. Mean cisplatin dose was 86 mg/m2 in the granisetron hydrochloride injection group and 80 mg/m2 in the placebo group.Table 3 Prevention of Chemotherapy-Induced Nausea and Vomiting-Single-Day Cisplatin Therapy1 Granisetron Hydrochloride Injection Placebo P-Value Number of Patients 14 14 Response Over 24 Hours Complete Response2 93% 7% <0.001 No Vomiting 93% 14% <0.001 No More Than Mild Nausea 93% 7% <0.001
2No vomiting and no moderate or severe nausea.
Granisetron hydrochloride injection was also evaluated in a randomized dose response study of cancer patients receiving cisplatin ≥75 mg/m2. Additional chemotherapeutic agents included: anthracyclines, carboplatin, cytostatic antibiotics, folic acid derivatives, methylhydrazine, nitrogen mustard analogs, podophyllotoxin derivatives, pyrimidine analogs, and vinca alkaloids. Granisetron hydrochloride injection doses of 10 and 40 mcg/kg were superior to 2 mcg/kg in preventing cisplatin-induced nausea and vomiting, but 40 mcg/kg was not significantly superior to 10 mcg/kg (see Table 4).
1Cisplatin administration began within 10 minutes of granisetron hydrochloride injection infusion and continued for 2.6 hours (mean). Mean cisplatin doses were 96 to 99 mg/m2.Table 4 Prevention of Chemotherapy-Induced Nausea and Vomiting-Single-Day High-Dose Cisplatin Therapy1 Granisetron Hydrochloride Injection
(mcg/kg)P-Value
(vs. 2 mcg/kg)2 10 40 10 40 Number of Patients 52 52 53 Response Over 24 Hours Complete Response2 31% 62% 68% <0.002 <0.001 No Vomiting 38% 65% 74% <0.001 <0.001 No More Than Mild Nausea 58% 75% 79% NS 0.007
2No vomiting and no moderate or severe nausea.
Granisetron hydrochloride injection was also evaluated in a double-blind, randomized dose response study of 353 patients stratified for high (≥80 to 120 mg/m2) or low (50 to 79 mg/m2) cisplatin dose. Response rates of patients for both cisplatin strata are given in Table 5.
1Cisplatin administration began within 10 minutes of granisetron hydrochloride injection infusion and continued for 2 hours (mean). Mean cisplatin doses were 64 and 98 mg/m2 for low and high strata.Table 5 Prevention of Chemotherapy-Induced Nausea and Vomiting-Single-Day High-Dose and Low-Dose Cisplatin Therapy1 Granisetron Hydrochloride Injection
(mcg/kg)P-Value
(vs. 5 mcg/kg)5 10 20 40 10 20 40 High-Dose Cisplatin Number of Patients 40 49 48 47 Response Over 24 Hours Complete Response2 18% 41% 40% 47% 0.018 0.025 0.004 No Vomiting 28% 47% 44% 53% NS NS 0.016 No Nausea 15% 35% 38% 43% 0.036 0.019 0.005 Low-Dose Cisplatin Number of Patients 42 41 40 46 Response Over 24 Hours Complete Response2 29% 56% 58% 41% 0.012 0.009 NS No Vomiting 36% 63% 65% 43% 0.012 0.008 NS No Nausea 29% 56% 38% 33% 0.012 NS NS
2No vomiting and no use of rescue antiemetic.
For both the low and high cisplatin strata, the 10, 20, and 40 mcg/kg doses were more effective than the 5 mcg/kg dose in preventing nausea and vomiting within 24 hours of chemotherapy administration. The 10 mcg/kg dose was at least as effective as the higher doses.
Moderately Emetogenic Chemotherapy
Granisetron hydrochloride injection, 40 mcg/kg, was compared with the combination of chlorpromazine (50 to 200 mg/24 hours) and dexamethasone (12 mg) in patients treated with moderately emetogenic chemotherapy, including primarily carboplatin >300 mg/m2, cisplatin 20 to 50 mg/m2 and cyclophosphamide >600 mg/m2. Granisetron hydrochloride injection was superior to the chlorpromazine regimen in preventing nausea and vomiting (see Table 6).
1Patients also received dexamethasone, 12 mg.Table 6 Prevention of Chemotherapy-Induced Nausea and Vomiting-Single-Day Moderately Emetogenic Chemotherapy Granisetron Hydrochloride Injection Chlorpromazine1 P-Value Number of Patients 133 133 Response Over 24 Hours Complete Response2 68% 47% <0.001 No Vomiting 73% 53% <0.001 No More Than Mild Nausea 77% 59% <0.001
2No vomiting and no moderate or severe nausea.
In other studies of moderately emetogenic chemotherapy, no significant difference in efficacy was found between granisetron hydrochloride injection doses of 40 mcg/kg and 160 mcg/kg.
Repeat-Cycle Chemotherapy
In an uncontrolled trial, 512 cancer patients received granisetron hydrochloride injection, 40 mcg/kg, prophylactically, for two cycles of chemotherapy, 224 patients received it for at least four cycles, and 108 patients received it for at least six cycles. Granisetron hydrochloride injection efficacy remained relatively constant over the first six repeat cycles, with complete response rates (no vomiting and no moderate or severe nausea in 24 hours) of 60% to 69%. No patients were studied for more than 15 cycles.
Pediatric Studies
A randomized double-blind study evaluated the 24-hour response of 80 pediatric cancer patients (age 2 to 16 years) to granisetron hydrochloride injection 10, 20 or 40 mcg/kg. Patients were treated with cisplatin ≥60 mg/m2, cytarabine ≥3 g/m2, cyclophosphamide ≥1 g/m2 or nitrogen mustard ≥6 mg/m2 (see Table 7).
1No vomiting and no moderate or severe nausea.Table 7 Prevention of Chemotherapy-Induced Nausea and Vomiting in Pediatric Patients Granisetron Hydrochloride Injection Dose (mcg/kg) 10 20 40 Number of Patients 29 26 25 Median Number of Vomiting Episodes 2 3 1 Complete Response Over 24 Hours1 21% 31% 32%
A second pediatric study compared granisetron hydrochloride injection 20 mcg/kg to chlorpromazine plus dexamethasone in 88 patients treated with ifosfamide ≥3 g/m2/day for two or three days. Granisetron hydrochloride injection was administered on each day of ifosfamide treatment. At 24 hours, 22% of granisetron hydrochloride injection patients achieved complete response (no vomiting and no moderate or severe nausea in 24 hours) compared with 10% on the chlorpromazine regimen. The median number of vomiting episodes with granisetron hydrochloride injection was 1.5; with chlorpromazine it was 7.0.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Granisetron hydrochloride injection USP, 1 mg/mL (free base), is supplied in 4 mL Multi-Use Vials. Contains preservative.
NDC: 64679-661-02 (package of 1 Multi-Use Vial)
Store at 20°-25°C (68°-77°F). [See USP Controlled Room Temperature]
Once the multi-use vial is penetrated, its contents should be used within 30 days.
Do not freeze. Protect from light.
-
17 PATIENT COUNSELING INFORMATION
Patients should be informed that the most common adverse reactions for the indication of chemotherapy induced nausea and vomiting are headache and constipation (see Table 1).
Patients should be advised of the risk of allergic reactions if they have a prior allergic reaction to a class of antiemetics known as 5-HT3 receptor antagonists.
Electrocardiogram changes (QT prolongation) have been reported with the use of granisetron hydrochloride injection. Patients should be cautioned about the use of this drug if they have heart problems or take medications for heart problems.
Patients should be informed that granisetron hydrochloride injection, USP 1 mg/mL contains methylparaben and propylparaben.
Manufactured by:
Wockhardt Limited
Mumbai, India.
Distributed by:
Wockhardt USA LLC.
20 Waterview Blvd.
Parsippany, NJ 07054
USA.
Rev.080811 - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GRANISETRON HYDROCHLORIDE
granisetron hydrochloride injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 55648-661 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GRANISETRON HYDROCHLORIDE (UNII: 318F6L70J8) (GRANISETRON - UNII:WZG3J2MCOL) GRANISETRON 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 55648-661-02 1 in 1 CARTON 1 4 mL in 1 VIAL, MULTI-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078565 06/30/2008 Labeler - WOCKHARDT LIMITED (650069115) Establishment Name Address ID/FEI Business Operations WOCKHARDT LIMITED 676257570 manufacture
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.

