Indagel Max Hand Sanitizer
Indagel Max Hand Sanitizer by
Drug Labeling and Warnings
Indagel Max Hand Sanitizer by is a Otc medication manufactured, distributed, or labeled by INDALABOR INDAIA LABORATORIO FARMACEUTICO LTDA. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
INDAGEL MAX HAND SANITIZER- alcohol gel
INDALABOR INDAIA LABORATORIO FARMACEUTICO LTDA
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
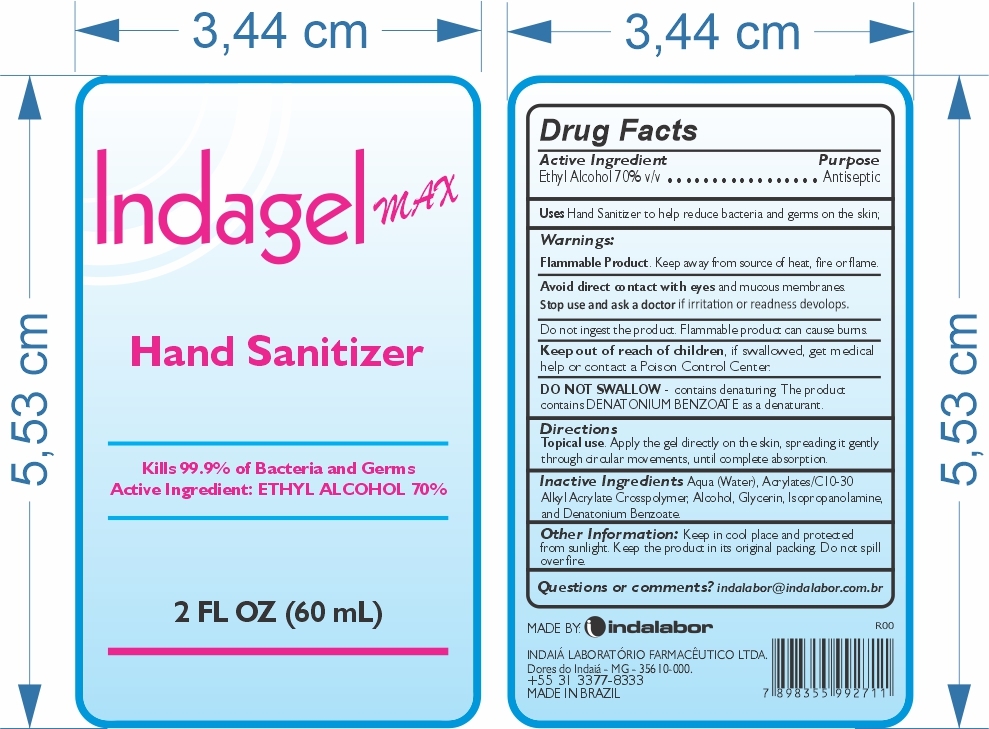
Indagel Max Hand Sanitizer
Warnings:
Flammable Product. Keep away from source of heat, fire, or flame.
Directions
Topical use. Apply the gel directly on the skin, spreading it gently through circular movements, until complete absorption.
Inactive Ingredients
Aqua (water), Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Alcohol, Glycerin, Isopropanolamine, and Denatonium Benzoate.
Other Information.
Keep in cool place and protected from sunlight. Keep the product in its original packing. Do not spill over fire.
Package 60ml/100ml/250ml/500ml
MADE BY: INDALABOR
INDAIÁ LABORATÓRIO FARMACÊUTICO LTDA
Dores do Indaiá, MG - 35610-000
+55 31 3377-8833
MADE IN BRAZIL
NDC: 80342-000-01
NDC: 80342-000-02

NDC: 80342-000-03

NDC: 80342-000-04

| INDAGEL MAX HAND SANITIZER
alcohol gel |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - INDALABOR INDAIA LABORATORIO FARMACEUTICO LTDA (897123209) |
| Registrant - INDALABOR INDAIA LABORATORIO FARMACEUTICO LTDA (897123209) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.