ANTISEPTIC HAND RUB- alcohol liquid
ChemQuest Chemicals LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
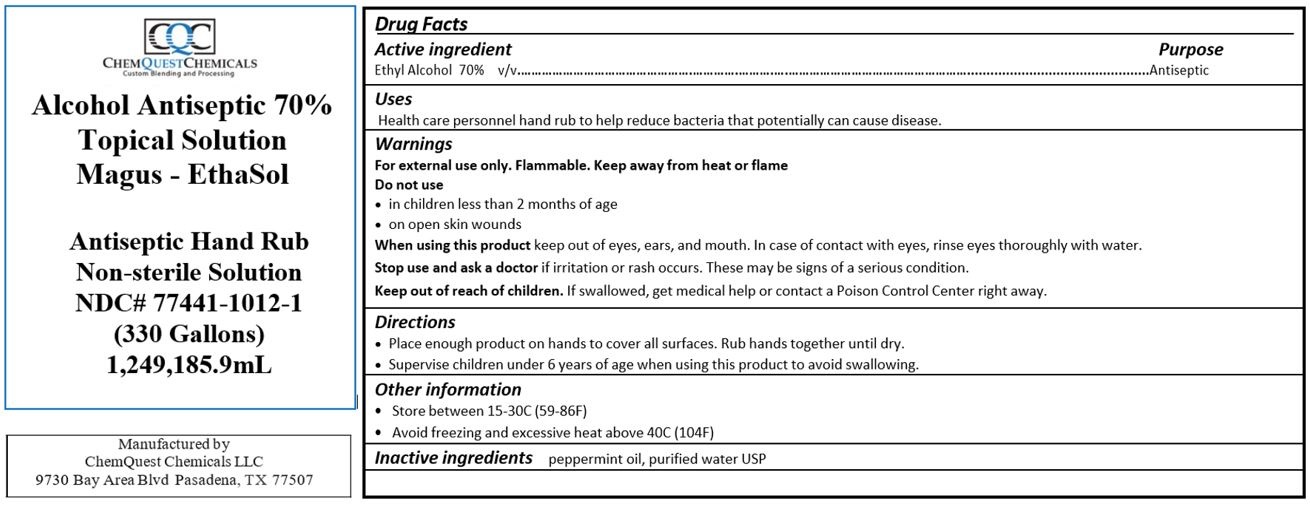
Alcohol Antisptic 70% Topical Solution Magus-EthaSol
Purpose
Antiseptic, Hand Rub
Drug Facts
| Active Ingredient | Purpose |
| Alcohol 70% v/v. | Antiseptic |
Uses
Health care personnel hand rub to help reduce bacteria that potentially can cause disease.
Warnings
For external use only. Flammable. Keep away from heat or flame
Do not use
- in children less than 2 months of age
- on open skin wounds
When using this product keep out of eyes, ears, and mouth. In case of contact with eyes, rinse eyes thoroughly with water.
Stop use and ask a doctor if irritation or rash occurs. These may be signs of a serious condition.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Place enough product on hands to cover all surfaces. Rub hands together until dry.
- Supervise children under 6 years of age when using this product to avoid swallowing.
Other information
- Store between 15-30C (59-86F)
- Avoid freezing and excessive heat above 40C (104F)
Inactive ingredients
peppermint oil, purified water USP
Package Label - Principal Display Panel
1249.1859 L NDC: 77441-1012-1
ANTISEPTIC HAND RUB
alcohol liquid |
| Product Information |
| Product Type | HUMAN OTC DRUG | Item Code (Source) | NDC: 77441-1012 |
| Route of Administration | TOPICAL |
|
| Active Ingredient/Active Moiety |
| Ingredient Name | Basis of Strength | Strength |
| ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) | ALCOHOL | 7000 L in 10000 L |
|
| Inactive Ingredients |
| Ingredient Name | Strength |
| PEPPERMINT OIL (UNII: AV092KU4JH) | |
| WATER (UNII: 059QF0KO0R) | |
|
|
| Packaging |
| # | Item Code | Package Description | Marketing Start Date | Marketing End Date |
| 1 | NDC: 77441-1012-1 | 1249.1859 L in 1 CONTAINER; Type 0: Not a Combination Product | 09/14/2020 | |
|
|
| Marketing Information |
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph not final | part333A | 09/14/2020 | 08/18/2022 |
|
| Labeler - ChemQuest Chemicals LLC
(835052051)
|
