BISACODYL- bisacodyl suppository
Bisacodyl by
Drug Labeling and Warnings
Bisacodyl by is a Otc medication manufactured, distributed, or labeled by CARDINAL HEALTH, INC., G&W Laboratories, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT (in each suppository)
- PURPOSE
- USES
-
WARNINGS
For rectal use only
Do not use
laxative products for a period longer than one week unless directed by a doctor
Ask a doctor before use if you have
abdominal pain, nausea or vomiting
a sudden change in bowel habits that lasts longer than 2 weeks
Stop use and ask a doctor
if rectal bleeding occurs or you fail to have a bowel movement after using a laxative. This may indicate a serious condition. - IF PREGNANT OR BREAST FEEDING,
- KEEP OUT OF REACH OF CHILDREN SECTION
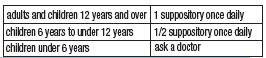
- DIRECTIONS
- OTHER INFORMATION
- INACTIVE INGREDIENT
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC: 37205-102-53
LEADER®
BISACODYL SUPPOSITORIES
LAXATIVE
FOR PROMPT RELIEF OF CONSTIPATION
12 BISACODYL SUPPOSITORIES 10 MG EACH
Compare to Dulcolax® active ingredient*
Satisfaction Guaranteed

-
INGREDIENTS AND APPEARANCE
BISACODYL
bisacodyl suppositoryProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 37205-102 Route of Administration RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Bisacodyl (UNII: 10X0709Y6I) (Bisacodyl - UNII:10X0709Y6I) Bisacodyl 10 mg Inactive Ingredients Ingredient Name Strength Hydrogenated Palm Kernel Oil (UNII: FM8D1RE2VP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 37205-102-53 12 in 1 CARTON Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part334 09/10/1997 Labeler - CARDINAL HEALTH, INC. (097537435) Registrant - G&W Laboratories, Inc. (001271188) Establishment Name Address ID/FEI Business Operations G&W Laboratories, Inc. 001271188 MANUFACTURE
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.