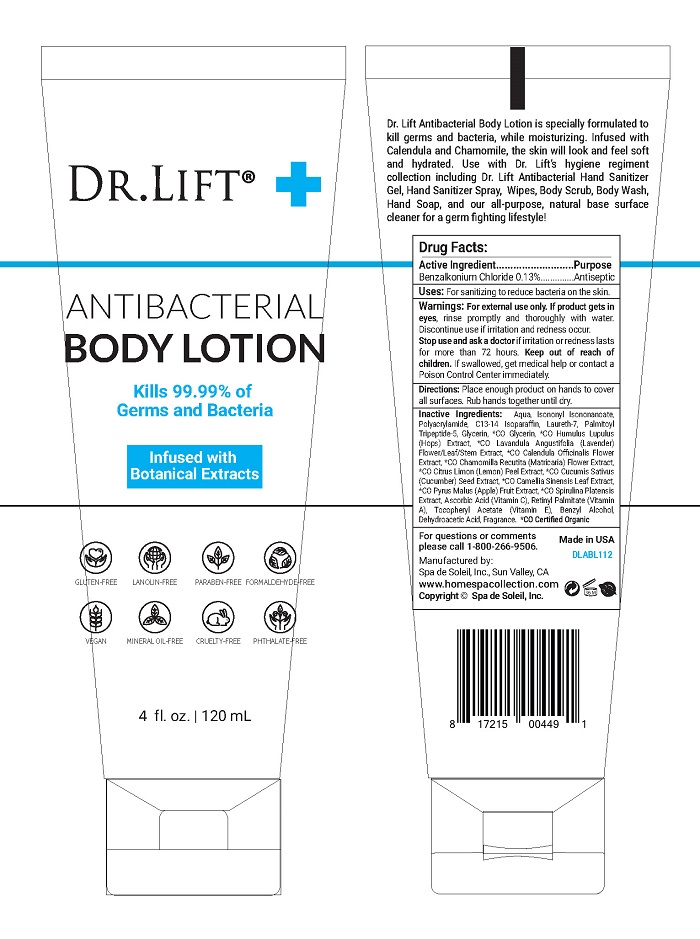
DR LIFT ANTIBACTERIAL- benzalkonium chloride lotion
Dr Lift Antibacterial by
Drug Labeling and Warnings
Dr Lift Antibacterial by is a Otc medication manufactured, distributed, or labeled by Spa de Soleil. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredient
- Purpose
-
Warnings
Warnings
For external use only. If product gets in eyes, rinse promptly and thoroughly with water. Discontinue use if irritation and redness occur.
Stop use and ask a doctor if irritation or redness lasts for more than 72 hours. Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately.
- KEEP OUT OF REACH OF CHILDREN
- Directions
-
Inactive Ingredients
Inactive Ingredients:
Aqua, Isononyl Isononanoate, Polyacrylamide, C13-14 Isoparaffin, Laureth-7, Palmitoyl Tripeptide-5, Glycerin, *CO Glycerin, *CO Humulus Lupulus (Hops) Extract, *CO Lavandula Angustifolia (Lavender) Flower/Leaf/Stem Extract, *CO Calendula Officinalis Flower Extract, *CO Chamomilla Recutita (Matricaria) Flower Extract, *CO Citrus Limon (Lemon) Peel Extract, *CO Cucumis Sativus (Cucumber) Seed Extract, *CO Camellia Sinensis Leaf Extract, *CO Pyrus Malus (Apple) Fruit Extract, *CO Spirulina Platensis Extract, Ascorbic Acid (Vitamin C), Retinyl Palmitate (Vitamin A), Tocopheryl Acetate (Vitamin E), Benzyl Alcohol, Dehydroacetic Acid, Fragrance. *CO Certified Organic
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DR LIFT ANTIBACTERIAL
benzalkonium chloride lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 68062-2243 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.156 mg in 120 mg Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68062-2243-1 120 mg in 1 BOTTLE; Type 0: Not a Combination Product 09/17/2020 2 NDC: 68062-2243-2 100 mg in 1 BOTTLE; Type 0: Not a Combination Product 10/10/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 09/17/2020 Labeler - Spa de Soleil (874682867) Establishment Name Address ID/FEI Business Operations Spa de Soleil 874682867 manufacture(68062-2243)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.