MYXREDLIN- insulin human injection, solution
MYXREDLIN by
Drug Labeling and Warnings
MYXREDLIN by is a Prescription medication manufactured, distributed, or labeled by Baxter Healthcare Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use MYXREDLIN safely and effectively. See full prescribing information for MYXREDLIN.
MYXREDLIN (insulin human in sodium chloride injection), for intravenous use
Initial U.S. Approval: 1991INDICATIONS AND USAGE
MYXREDLIN is a short-acting human insulin indicated to improve glycemic control in adults and pediatric patients with diabetes mellitus (1).
DOSAGE AND ADMINISTRATION
- Inspect MYXREDLIN visually before use. It should appear clear and colorless. Do not use MYXREDLIN if particulate matter or coloration is seen. (2.1)
- Administer MYXREDLIN intravenously ONLY under medical supervision with close monitoring of blood glucose and potassium levels.(2.1)
- Do not add supplementary medication or additives. (2.1)
- Do not use in series connections. (2.1)
- Do not shake or freeze. Discard unused portion. (2.1)
- Individualize dose based on metabolic needs, blood glucose monitoring results, and glycemic control goal. (2.2)
- Dosage adjustments may be needed with changes in nutrition, renal, or hepatic function or during acute illness. (2.2)
DOSAGE FORMS AND STRENGTHS
Injection: 100 units insulin human in 100 mL of 0.9% sodium chloride (1 unit/mL) in a single-dose container (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Hyper- or Hypoglycemia with Changes in Insulin Regimen: Carry out under close medical supervision and increase frequency of blood glucose monitoring. (5.1)
- Hypoglycemia: May be life-threatening. Factors which may increase the risk include changes in nutrition and co-administered medication and patients with renal or hepatic impairment. Increased frequency of blood glucose monitoring is recommended in patients at increased risk. (5.2)
- Hypersensitivity Reactions: Severe, life-threatening, generalized allergy, including anaphylaxis, can occur. Discontinue MYXREDLIN, monitor, and treat if indicated. (5.3)
- Hypokalemia: May be life-threatening. Monitor potassium levels and treat if indicated. (5.4)
- Fluid Retention and Heart Failure with Concomitant Use of Thiazolidinediones (TZDs): Observe for signs and symptoms of heart failure; consider dosage reduction or discontinuation if heart failure occurs. (5.5)
ADVERSE REACTIONS
Adverse reactions observed with insulin human injection include hypoglycemia, allergic reactions, weight gain and edema (6).
To report SUSPECTED ADVERSE REACTIONS, contact Baxter Healthcare at 1-866-888-2472 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Drugs that may increase the risk of hypoglycemia: antidiabetic agents, ACE inhibitors, angiotensin II receptor blocking agents, disopyramide, fibrates, fluoxetine, monoamine oxidase inhibitors, pentoxifylline, pramlintide, salicylates, somatostatin analog (e.g., octreotide), and sulfonamide antibiotics. (7)
- Drugs that may decrease the blood glucose lowering effect: atypical antipsychotics, corticosteroids, danazol, diuretics, estrogens, glucagon, isoniazid, niacin, oral contraceptives, phenothiazines, progestogens (e.g., in oral contraceptives), protease inhibitors, somatropin, sympathomimetic agents (e.g., albuterol, epinephrine, terbutaline), and thyroid hormones. (7)
- Drugs that may increase or decrease the blood glucose lowering effect: Alcohol, beta-blockers, clonidine, lithium salts, and pentamidine. (7)
- Drugs that may blunt the signs and symptoms of hypoglycemia: beta-blockers, clonidine, guanethidine, and reserpine. (7)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 6/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Dosage Information
2.3 Dosage Adjustment due to Drug Interactions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hyperglycemia or Hypoglycemia with Changes in Insulin Regimen
5.2 Hypoglycemia
5.3 Hypersensitivity and Allergic Reactions
5.4 Hypokalemia
5.5 Fluid Retention and Heart Failure with Concomitant Use of PPAR-gamma agonists
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
- Inspect MYXREDLIN visually before use. It should appear clear and colorless. Do not use MYXREDLIN if particulate matter or coloration is seen.
- Administer MYXREDLIN intravenously ONLY under medical supervision with close monitoring of blood glucose and potassium levels [see Warnings and Precautions (5.2, 5.4)].
- Do not add supplementary medication or additives.
- Do not use in series connections.
- Do not shake. Do not freeze. Discard any unused portion.
2.2 Dosage Information
- Individualize and adjust the dosage of MYXREDLIN based on the individual's metabolic needs, blood glucose monitoring results, and glycemic control goal.
- Dosage adjustments may be needed with changes in nutrition, changes in renal or hepatic function or during acute illness [see Warnings and Precautions (5.1, 5.2) and Use in Specific Populations (8.6, 8.7)].
2.3 Dosage Adjustment due to Drug Interactions
- Dosage adjustment may be needed when MYXREDLIN is used concomitantly with certain drugs [see Drug Interactions (7)].
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hyperglycemia or Hypoglycemia with Changes in Insulin Regimen
Changes in insulin strength, manufacturer, type, or method of administration may affect glycemic control and predispose to hypoglycemia [see Warnings and Precautions (5.2)] or hyperglycemia. These changes should be made cautiously and under close medical supervision and the frequency of blood glucose monitoring should be increased.
5.2 Hypoglycemia
Hypoglycemia is the most common adverse reaction of all insulins, including MYXREDLIN. Severe hypoglycemia can cause seizures, may lead to unconsciousness, may be life threatening or cause death. Hypoglycemia can impair concentration ability and reaction time.
Hypoglycemia can happen suddenly and symptoms may differ in each individual and change over time in the same individual. Symptomatic awareness of hypoglycemia may be less pronounced in patients with longstanding diabetes, in patients with diabetic nerve disease, in patients using medications that block the sympathetic nervous system (e.g., beta-blockers) [see Drug Interactions (7)], or in patients who experience recurrent hypoglycemia.
Risk Factors for Hypoglycemia
Factors which may increase the risk of hypoglycemia include changes in nutrition and co-administered medication [see Drug Interactions (7)]. Patients with renal or hepatic impairment may be at higher risk of hypoglycemia [see Use in Specific Populations (8.6, 8.7)].Risk Mitigation Strategies for Hypoglycemia
Patients and caregivers must be educated to recognize hypoglycemia. In patients at higher risk for hypoglycemia and patients who have reduced symptomatic awareness of hypoglycemia, increased frequency of blood glucose monitoring is recommended.5.3 Hypersensitivity and Allergic Reactions
Severe, life-threatening, generalized allergy, including anaphylaxis, can occur with MYXREDLIN. Generalized allergy to insulin may manifest as a whole body rash (including pruritus), dyspnea, wheezing, hypotension, tachycardia, or diaphoresis. If hypersensitivity reactions occur, discontinue MYXREDLIN; treat per standard of care and monitor until symptoms and signs resolve. MYXREDLIN is contraindicated in patients who have had hypersensitivity reactions to insulin human or any of the excipients in MYXREDLIN [see Contraindications (4)].
5.4 Hypokalemia
All insulins, including MYXREDLIN, cause a shift in potassium from the extracellular to intracellular space, possibly leading to hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Monitor potassium levels and treat if indicated.
5.5 Fluid Retention and Heart Failure with Concomitant Use of PPAR-gamma agonists
Thiazolidinediones (TZDs), which are peroxisome proliferator-activated receptor (PPAR)-gamma agonists, can cause dose-related fluid retention, particularly when used in combination with insulin. Fluid retention may lead to or exacerbate heart failure. Patients treated with insulin, including MYXREDLIN, and a PPAR-gamma agonist should be observed for signs and symptoms of heart failure. If heart failure develops, it should be managed according to current standards of care, and discontinuation or dose reduction of the PPAR-gamma agonist must be considered.
-
6 ADVERSE REACTIONS
The following adverse reactions are also discussed elsewhere in the labeling:
- Hypoglycemia [see Warnings and Precautions (5.2)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.3)]
- Hypokalemia [see Warnings and Precautions (5.4)]
Adverse Reactions from Clinical Studies or Postmarketing Reports
The following additional adverse reactions have been identified during clinical studies or from postmarketing reports with use of insulin human injection. Because some of these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or to establish a causal relationship to drug exposure.Adverse reactions associated with insulin initiation and glucose control intensification
Intensification or rapid improvement in glucose control has been associated with a transitory, reversible ophthalmologic refraction disorder, worsening of diabetic retinopathy, and acute painful peripheral neuropathy. Over the long-term, improved glycemic control decreases the risk of diabetic retinopathy and neuropathy.Hypersensitivity reactions
Severe, life-threatening, generalized allergy, including anaphylaxis.Hypoglycemia
Hypoglycemia is the most commonly observed adverse reaction with MYXREDLIN.Hypokalemia
MYXREDLIN can cause a shift in potassium from the extracellular to intracellular space, possibly leading to hypokalemia.Peripheral edema
Insulins, including MYXREDLIN, may cause sodium retention and edema, particularly if previously poor metabolic control is improved by intensified insulin therapy.Weight gain
Weight gain can occur with insulin therapies, including MYXREDLIN, and has been attributed to the anabolic effects of insulin and the decrease in glucosuria.Immunogenicity
As with all therapeutic peptides, insulin administration may cause anti-insulin antibodies to form. Increases in titers of anti-insulin antibodies that react with human insulin have been observed in patients treated with subcutaneous insulin human injection. -
7 DRUG INTERACTIONS
Table 1: Clinically Significant Drug Interactions with MYXREDLIN Drugs that May Increase the Risk of Hypoglycemia
Drugs:
Antidiabetic agents, ACE inhibitors, angiotensin II receptor blocking agents, disopyramide, fibrates, fluoxetine, monoamine oxidase inhibitors, pentoxifylline, pramlintide, salicylates, somatostatin analog (e.g., octreotide), and sulfonamide antibiotics.
Intervention:
Dose adjustment and increased frequency of glucose monitoring may be required when MYXREDLIN is co-administered with these drugs.
Drugs that May Decrease the Blood Glucose Lowering Effect of MYXREDLIN
Drugs:
Atypical antipsychotics (e.g., olanzapine and clozapine), corticosteroids, danazol, diuretics, estrogens, glucagon, isoniazid, niacin, oral contraceptives, phenothiazines, progestogens (e.g., in oral contraceptives), protease inhibitors, somatropin, sympathomimetic agents (e.g., albuterol, epinephrine, terbutaline), and thyroid hormones.
Intervention:
Dose adjustment and increased frequency of glucose monitoring may be required when MYXREDLIN is co-administered with these drugs.
Drugs that May Increase or Decrease the Blood Glucose Lowering Effect of MYXREDLIN
Drugs:
Alcohol, beta-blockers, clonidine, and lithium salts. Pentamidine may cause hypoglycemia, which may sometimes be followed by hyperglycemia.
Intervention:
Dose adjustment and increased frequency of glucose monitoring may be required when MYXREDLIN is co-administered with these drugs.
Drugs that May Blunt Signs and Symptoms of Hypoglycemia
Drugs:
Beta-blockers, clonidine, guanethidine, and reserpine.
Intervention:
Increased frequency of glucose monitoring may be required when MYXREDLIN is co-administered with these drugs.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from published studies over decades have not established an association with human insulin use during pregnancy and major birth defects, miscarriage or adverse maternal or fetal outcomes (see Data). There are risks to the mother and fetus associated with poorly controlled diabetes in pregnancy (see Clinical Considerations). Animal reproduction studies were not performed.The estimated background risk of major birth defects is 6-10% in women with pre-gestational diabetes with a HbA1c >7 and has been reported to be as high as 20-25% in women with a HbA1c >10. The estimated background risk of miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal riskPoorly controlled diabetes in pregnancy increases the maternal risk for diabetic ketoacidosis, preeclampsia, spontaneous abortions, preterm delivery, and delivery complications. Poorly controlled diabetes increases the fetal risk for major birth defects, stillbirth, and macrosomia-related morbidity.
Data
Human DataWhile available studies cannot definitively establish the absence of risk, published data from retrospective studies, open-label, randomized, parallel studies and meta-analyses have not established an association with human insulin use during pregnancy and major birth defects, miscarriage, or adverse maternal or fetal outcomes. All available studies have methodological limitations including lack of blinding, unclear methods of randomization, and small sample size.
8.2 Lactation
Risk Summary
Available data from published literature suggest that exogenous human insulin products, including insulin human injection, are transferred into human milk. There are no adverse reactions reported in the breastfed infants in the literature. There are no data on the effects of exogenous human insulin products, including MYXREDLIN, on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for MYXREDLIN and any potential adverse effects on the breastfed infant from MYXREDLIN or from the underlying maternal condition.8.4 Pediatric Use
MYXREDLIN is indicated to improve glycemic control in pediatric patients with diabetes mellitus.
The dosage of MYXREDLIN must be individualized in pediatric patients based on metabolic needs and frequent monitoring of blood glucose to reduce the risk of hypoglycemia [see Dosage and Administration (2.2) and Warnings and Precautions (5.2)].
8.5 Geriatric Use
The effect of age on the pharmacokinetics and pharmacodynamics of insulin human injection has not been studied.
Elderly patients using MYXREDLIN, may be at increased risk of hypoglycemia due to co-morbid disease [see Warnings and Precautions (5.2)].
8.6 Renal Impairment
The effect of renal impairment on the pharmacokinetics and pharmacodynamics of MYXREDLIN has not been studied. Patients with renal impairment are at increased risk of hypoglycemia and may require more frequent MYXREDLIN dose adjustment and more frequent blood glucose monitoring [see Warnings and Precautions (5.2)].
8.7 Hepatic Impairment
The effect of hepatic impairment on the pharmacokinetics and pharmacodynamics of MYXREDLIN has not been studied. Patients with hepatic impairment are at increased risk of hypoglycemia and may require more frequent MYXREDLIN dose adjustment and more frequent blood glucose monitoring [see Warnings and Precautions (5.2)].
-
10 OVERDOSAGE
Excess insulin administration may cause hypoglycemia and hypokalemia. More severe episodes with coma, seizure, or neurologic impairment can be treated with intramuscular or subcutaneous glucagon or intravenous glucose. Sustained monitoring may be necessary because hypoglycemia may recur after apparent clinical recovery. Hypokalemia must be corrected appropriately. [see Warnings and Precautions (5.2, 5.4)]
-
11 DESCRIPTION
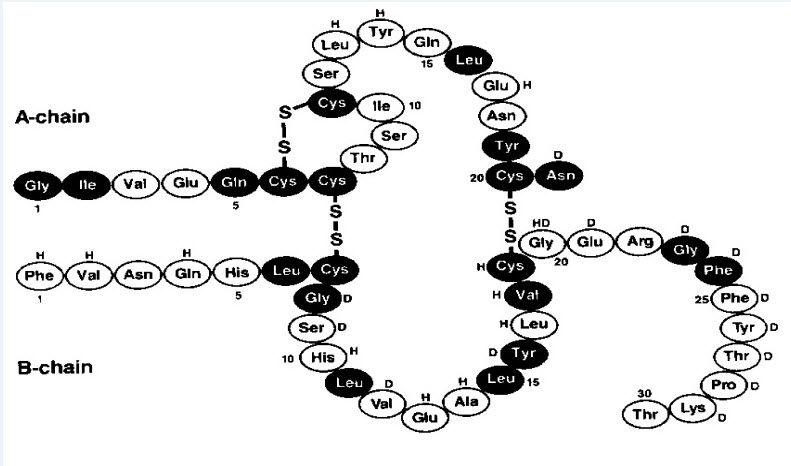
Insulin human is a short-acting human insulin. It is a polypeptide hormone and is produced by recombinant DNA technology, utilizing Pichia pastoris (a yeast) as the production organism. Insulin human is regular human insulin and has the empirical formula C257H383N65O77S6 and a molecular weight of 5808.
Figure 1: Structural formula of Insulin Human
MYXREDLIN (insulin human in sodium chloride injection) for intravenous use is a sterile, preservative-free, nonpyrogenic, clear, aqueous, and colorless solution supplied in a 100 mL GALAXY single-dose container. MYXREDLIN contains 100 units of insulin human in 100 milliliters of 0.9% sodium chloride injection. Each milliliter of solution contains 1 unit Insulin Human, USP; 0.412 mg Dibasic Sodium Phosphate Anhydrous, USP; 0.29 mg Monobasic Sodium Phosphate Monohydrate, USP; 9 mg Sodium Chloride, USP; and Water for Injection, USP. The pH range is 6.5-7.2.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The primary activity of insulin, including MYXREDLIN is the regulation of glucose metabolism. Insulins lower blood glucose by stimulating peripheral glucose uptake, especially by skeletal muscle and fat, and by inhibiting hepatic glucose production. Insulin inhibits lipolysis and proteolysis, and enhances protein synthesis.
12.2 Pharmacodynamics
MYXREDLIN is a short-acting insulin. The time course of insulin action (i.e., glucose lowering) may vary considerably in different individuals, within the same individual, and different doses. In a double-blind, randomized, crossover, euglycemic glucose clamp study described below, the average onset of action, defined as start of intravenous glucose infusion during the clamp, was approximately 21 minutes after starting of the intravenous infusion administration. The glucose infusion rate gradually increased to a maximum response of 13.7 mg/kg/min after 5 hours of human insulin infusion.
12.3 Pharmacokinetics
In a double-blind, randomized, crossover, euglycemic glucose clamp study, fifty eight healthy male subjects between 19 and 50 years of age received an intravenous infusion of either MYXREDLIN or another human insulin at 1 milliUnit/kg/min for 6 hours (0.36 units/kg total dose). On average, insulin concentrations of about 300 pM were attained approximately from 1.5 hours to 6 hours after starting intravenous infusion of MYXREDLIN, followed by a return to the baseline level 1.5 hours after stopping intravenous infusion.
Metabolism and Elimination
The mean terminal half-life from concentration data after stopping the intravenous infusion was estimated to be 23.4 minutes in healthy volunteers.
Specific Population
The effects of gender, age, obesity, renal and hepatic impairment on the pharmacodynamics and pharmacokinetics of insulin human injection have not been studied.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Standard 2-year carcinogenicity studies in animals have not been performed to evaluate the carcinogenic potential of insulin human injection.
Human insulin is not mutagenic in the following in vitro tests: The chromosomal aberration assay in human lymphocytes, the micronucleus assay in mouse polychromatic erythrocytes, and the mutation frequency assay in Chinese hamster cells.
Standard reproduction and teratology studies in animals, including fertility assessments have not been conducted with insulin human injection.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
MYXREDLIN (insulin human in sodium chloride injection) contains 100 units/100 mL (1 unit/mL) of insulin human in 0.9% sodium chloride and is a clear, colorless solution available as:
- 100 mL single-dose GALAXY container, package of 12, NDC: 0338-0126-12
16.2 Storage and Handling
Store MYXREDLIN in the refrigerator (36°F to 46°F [2°C to 8°C]) in the original carton to protect from light until administration. Do not use after the expiration date printed on the carton and container label.
If needed, MYXREDLIN may be stored at room temperature up to 77°F (25°C) for up to 30 days in the original carton. Once stored at room temperature, do not place back in the refrigerator. Discard MYXREDLIN after 30 days if stored at room temperature.
Do not freeze and do not use MYXREDLIN if it has been frozen. Do not shake.
-
17 PATIENT COUNSELING INFORMATION
Hypoglycemia
Inform patients that hypoglycemia is the most common adverse reaction with insulin. Inform patients that their ability to concentrate and react may be impaired as a result of hypoglycemia.
Hypersensitivity Reactions
Advise patients that hypersensitivity reactions have occurred with insulin human injection [see Warnings and Precautions (5.3)].
- SPL UNCLASSIFIED SECTION
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC: 0338-0126-12
MYXREDLIN
Insulin Human
in 0.9% Sodium Chloride Injection100 units per 100 mL
(1 unit / mL)100 mL Single-Dose GALAXY container
Discard unused portion
For Intravenous Infusion OnlyInsulin Human (Regular)
Sterile NonpyrogenicCautions: Do not add supplemental medication or additives.
Must not be used in series connections. Check for minute leaks
and solution clarity.
Each mL contains: 1 unit Insulin Human, USP; 0.412 mg Dibasic Sodium
Phosphate Anhydrous, USP; 0.29 mg Monobasic Sodium Phosphate
Monohydrate, USP; 9 mg Sodium Chloride, USP; and Water for Injection,
USP.
Dosage: See prescribing information.
Rx only
Store refrigerated at 36°F to 46°F (2°C to 8°C) in the original carton to
protect from light until administration. Do Not Shake. Do Not Freeze.
If needed, may store at room temperature up to 77°F (25°C) up
to 30 days in the original carton. Discard after 30 days if stored at
room temperature.
Baxter logo
Baxter, Galaxy and Myxredlin are trademarks of Baxter International Inc.
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Made in USA
Code 2G3322
PL 2501 Plastic07-34-00-0384
For Intravenous Infusion Only
MYXREDLIN
Insulin Human
in 0.9% Sodium Chloride Injection
100 units per 100 mL
(1 unit/mL)
NDC: 0338-0126-12
MYXREDLIN
Insulin Human
in 0.9% Sodium Chloride Injection
(1 unit/mL)UNVARNISHED AREA FOR ON-LINE
PRINTING OF LOT AND EXPUPC-A
Bar Code
PlacementSterile Nonpyrogenic
Each mL contains: 1 unit Insulin Human, USP; 0.412 mg Dibasic Sodium Phosphate Anhydrous,
USP; 0.29 mg Monobasic Sodium Phosphate Monohydrate, USP; 9 mg Sodium Chloride, USP;
and Water for Injection, USP.
Dosage: For Intravenous Infusion Only. See prescribing information.
Caution: Do not add supplemental medication or additives.
Rx only
Store refrigerated at 36°F to 46°F (2°C to 8°C) in the original carton to protect from light
until administration. Do Not Shake. Do Not Freeze.
If needed, may store MYXREDLIN at room temperature up to 77°F (25°C) up to 30 days in
the original carton. Once stored at room temperature, do not place back in the refrigerator.
Discard after 30 days if stored at room temperature.
Discard 30 days after storing at room temperature. Discard After
(Month) (Day) (Year)
(to be completed by dispensing pharmacist)
Baxter, Galaxy and Myxredlin are trademarks of Baxter International Inc.
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Made in USA
07-01-00-0167
NDC: 0338-0126-12
Code 2G3322MYXREDLIN
Insulin Human
in 0.9% Sodium Chloride Injection
100 units per 100 mL
(1 unit/mL)
For Intravenous Infusion Only
Insulin Human (REGULAR)USE CARTON TO PROTECT CONTENTS
FROM LIGHT UNTIL ADMINISTRATIONRx Only
1 Single-Dose Galaxy container
Discard unused portionBaxter Logo
MYXREDLIN
Insulin Human
in 0.9% Sodium Chloride Injection
100 units per 100 mL
(1 unit/mL)EXP: JUN 21
LOT #: NC125840
Bar Code
(01)50803380126120(17)210630(21)100000002582(10)NC125840Contains: 12 x 100 mL Single-Dose GALAXY Containers
NDC: 0338-0126-12
Code 2G3322
Store refrigerated at 36°F to 46°F (2°C to 8°C) Do Not Shake. Do Not Freeze.If needed, may store at room temperature up to 77°F (25°C) up to 30 days.
Once stored at room temperature, do not place back in the refrigerator.
Discard after 30 days if stored at room temperature.
Bar Code
GTIN 50303380126120
SERIAL 100000002582
EXP 210630
LOT NC125840Rx Only
Baxter Logo
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
07-06-00-0789G
-
INGREDIENTS AND APPEARANCE
MYXREDLIN
insulin human injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0338-0126 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INSULIN HUMAN (UNII: 1Y17CTI5SR) (INSULIN HUMAN - UNII:1Y17CTI5SR) INSULIN HUMAN 1.00 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) 0.412 mg in 1 mL SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) 0.29 mg in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 9 mg in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0338-0126-12 12 in 1 CARTON 07/12/2019 1 100 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA208157 07/12/2019 Labeler - Baxter Healthcare Corporation (005083209) Establishment Name Address ID/FEI Business Operations Baxter Healthcare Corporation 194684502 ANALYSIS(0338-0126) , MANUFACTURE(0338-0126) , LABEL(0338-0126) , PACK(0338-0126) , STERILIZE(0338-0126)
Trademark Results [MYXREDLIN]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MYXREDLIN 87287569 5903597 Live/Registered |
Baxter International Inc. 2017-01-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.