After Burn by Adventure Ready Brands After Burn
After Burn by
Drug Labeling and Warnings
After Burn by is a Otc medication manufactured, distributed, or labeled by Adventure Ready Brands. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
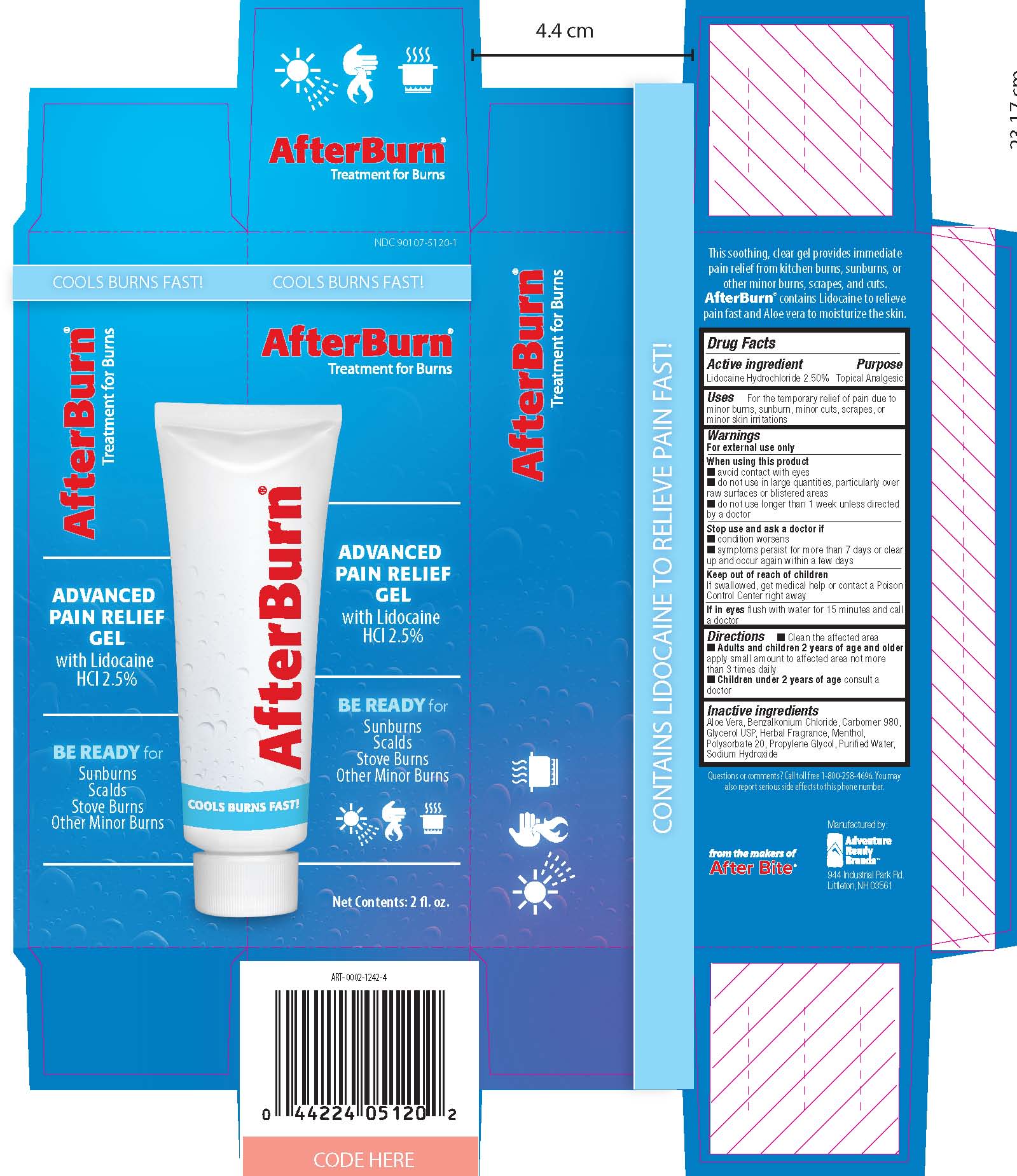
AFTER BURN- lidocaine hydrochloride gel
Adventure Ready Brands
----------
After Burn
Uses
For the temporary relief of pain due to minor burns, sunburn, minor cuts, screapes or minor skin irritations
When using this product
- avoid contact with eyes
- do not use in large quantities, particularly over raw surfaces or blistered areas
- do not use longer than 1 week unless directed by a doctor
Stop use and ask a doctor if
- condition worsens
- symptoms persist for more than 7 days or clear up and occur again within a few days
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
If in eyes, flush with water for 15 minutes and call a doctor.
Directions
Clean affected area
Adults and Children 2 years and older apply small amount to affected area not more than 3 times daily
Children under 2 years consult a doctor.
| AFTER BURN
lidocaine hydrochloride gel |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Adventure Ready Brands (064437304) |
| Registrant - Adventure Ready Brands (064437304) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Adventure Ready Brands | 064437304 | manufacture(90107-5120) | |
Revised: 12/2024
Document Id: 2a462e60-6356-f791-e063-6294a90a62e3
Set id: afd4fbe8-2b5d-300e-e053-2995a90a0c65
Version: 2
Effective Time: 20241227
Trademark Results [After Burn]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 AFTER BURN 90038548 not registered Live/Pending |
Geex Industries 1 2020-07-07 |
 AFTER BURN 87025787 5193214 Live/Registered |
Burncycle LLC 2016-05-05 |
 AFTER BURN 85495941 4386292 Live/Registered |
Tender Corporation 2011-12-15 |
 AFTER BURN 75823119 2481533 Dead/Cancelled |
Barretta, Richard 1999-10-15 |
 AFTER BURN 73762542 1556486 Dead/Cancelled |
WYATT CHEMICAL CORPORATION 1988-11-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.