ROMPUN- xylazine injection, solution
Rompun by
Drug Labeling and Warnings
Rompun by is a Animal medication manufactured, distributed, or labeled by Bayer HealthCare, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION:
Rompun® (xylazine injection) is supplied in 50 mL multiple dose vials as a sterile solution. Each mL contains 100 mg Rompun® (xylazine base equivalent); 0.9 mg methylparaben; 0.1 mg propylparaben; water for injection; citric acid and sodium citrate for pH adjustment to 5.5 ± 0.3.
ACTIVE INGREDIENT:
xylazine hydrochloride........................................................... 11.4%
(Equivalent to 10% base)INERT INGREDIENTS:........................................................... 88.6%
100.0% - CAUTION:
-
PHARMACOLOGY:
Rompun®, a non-narcotic compound, is a sedative and analgesic as well as muscle relaxant. Its sedative and analgesic activity is related to central nervous system depression. Its muscle-relaxant effect is based on inhibition of the intraneural transmission of impulses in the central nervous system. The principal pharmacological activities develop within 10 to 15 minutes after intramuscular injection, and within 3 to 5 minutes following intravenous administration in horses.
A sleeplike state, the depth of which is dose-dependent, is usually maintained for 1 to 2 hours, while analgesia lasts from 15 to 30 minutes. The centrally-acting muscle relaxant effect causes relaxation of the skeletal musculature, complementing sedation and analgesia.
In horses and Cervidae under the influence of Rompun® the respiratory rate is reduced as in natural sleep. Following treatment with Rompun® the heart rate is decreased and a transient change in the conductivity of the cardiac muscle may occur, as evidenced by a partial atrioventricular block. This resembles the atrioventricular block often observed in normal horses.1,2,3,4 Although a partial A-V block may occasionally occur following intramuscular injection of Rompun® (xylazine injection), the incidence is less than when it is administered intravenously. Intravenous administration of Rompun® (xylazine injection) causes a transient rise in blood pressure, followed by a slight decrease.
Rompun® has no effect on blood clotting time or other hematologic parameters.
-
INDICATIONS:
Rompun® (xylazine injection) should be used in horses and Cervidae (Fallow Deer, Mule Deer, Sika Deer, White-Tailed Deer and Elk) when it is desirable to produce a state of sedation accompanied by a shorter period of analgesia.
Horses: Rompun® (xylazine injection) has been used successfully as follows:
- 1. Diagnostic procedures–oral and ophthalmic examinations, abdominal palpation, rectal palpation, vaginal examination, catheterization of the bladder and radiographic examinations.
- 2. Orthopedic procedures, such as application of casting materials and splints.
- 3. Dental procedures.
- 4. Minor surgical procedures of short duration such as debridement, removal of cutaneous neoplasms and suturing of lacerations.
- 5. To calm and facilitate handling of fractious animals.
- 6. Therapeutic medication for sedation and relief of pain following injury or surgery.
- 7.
Major surgical procedures:
- a. When used as a preanesthetic to general anesthesia.
- b. When used in conjunction with local anesthetics.
Cervidae: Rompun® (xylazine injection) may be used for the following:
- 1. To calm and facilitate handling of fractious animals.
- 2. Diagnostic procedures.
- 3. Minor surgical procedures.
- 4. Therapeutic medication for sedation and relief of pain following injury or surgery.
- 5. As a preanesthetic to local anesthesia.
Rompun® at the recommended dosages can be used in conjunction with local anesthetics, such as procaine or lidocaine.
-
DOSAGE AND ADMINISTRATION:
- 1.
Horse Dosage:
Intravenously–0.5 mL/100 lbs body weight (0.5 mg/lb)
Intramuscularly–1.0 mL/100 lbs body weight (1.0 mg/lb)
Following injection of Rompun® (xylazine injection), the animal should be allowed to rest quietly until the full effect has been reached.
These dosages produce sedation which is usually maintained for 1 to 2 hours, and analgesia which lasts for 15 to 30 minutes.
- 2.
Preanesthetic to Local Anesthesia: Rompun® at the recommended dosages can be used in conjunction with local anesthetics, such as procaine or lidocaine.
- 3.
Preanesthetic to General Anesthesia: Rompun® at the recommended dosage rates, produces an additive effect to central nervous system depressants such as pentobarbital sodium, thiopental sodium and thiamylal sodium. Therefore, the dosage of such compounds should be reduced and administered to the desired effect. In general, only 1/3 to 1/2 of the calculated dosage of the barbiturates will be needed to produce a surgical plane of anesthesia. Post-anesthetic or emergence excitement has not been observed in animals preanesthetized with Rompun®.
Rompun® has been used successfully as a preanesthetic agent for pentobarbital sodium, thiopental sodium, thiamylal sodium, nitrous oxide, ether, halothane, glyceryl guaiacolate and methoxyflurane anesthesia.
- 4. Cervidae Dosage: Administer intramuscularly, either by hand syringe or syringe dart, in the heavy muscles of the croup or shoulder.
Cervidae Dosage Range:
Fallow Deer (Dama dama) - 2.0 to 4.0 mL/100 lbs body weight (2.0 to 4.0 mg/lb).
Mule Deer (Odocoileus hemionus) - 1.0 to 2.0 mL/100 lbs body weight (1.0 to 2.0 mg/lb).
Sika Deer (Cervus nippon) - 1.0 to 2.0 mL/100 lbs body weight (1.0 to 2.0 mg/lb)
White-Tailed Deer (Odocoileus virginianus) - 1.0 to 2.0 mL/100 lbs body weight (1.0 to 2.0 mg/lb).
Elk (Cervus canadensis) - 0.25 to 0.5 mL/100 lbs body weight (0.25 to 0.5 mg/lb).
Following injection of Rompun® the animal should be allowed to rest quietly until the full effect has been reached.
These dosages produce sedation which is usually maintained for 1 to 2 hours and analgesia which lasts for 15 to 30 minutes.
Use within 30 days of first puncture and puncture a maximum of 20 times. Any product remaining after 20 punctures or more than 30 days after initial puncture should be discarded.
- 1.
Horse Dosage:
-
SIDE EFFECTS:
Rompun® (xylazine injection), in horses and Cervidae, used at recommended dosage levels may occasionally cause slight muscle tremors, bradycardia with partial A-V heart block and a reduced respiratory rate. Movement in response to sharp auditory stimuli may be observed. In horses, sweating, rarely profuse, has been reported following administration. In Cervidae, salivation, various vocalizations (bellowing, bleating, groaning, grunting, snoring) on expiration, audible grinding of molar teeth, protruding tongue and elevated temperatures have also been noted in some cases.
-
PRECAUTIONS:
Careful consideration should be given before administering to horses and Cervidae with significantly depressed respiration, severe pathologic heart disease, advanced liver or kidney disease, severe endotoxic or traumatic shock, or stress conditions such as extreme heat, cold, high altitude or fatigue.
Do not use Rompun® in conjunction with tranquilizers.
Analgesic effect is variable, and depth should be carefully assayed prior to surgical/clinical procedures. Variability of analgesia occurs most frequently at the distal extremities of horses and Cervidae.
Horses: Since an additive effect results from the use of Rompun® and the barbiturate compounds, it should be used with caution with these central nervous system depressants. Products known to produce respiratory depression or apnea, such as thiamylal sodium, should be given at a reduced dosage and, when injected intravenously, should be administered slowly. When intravenous administration is desired, avoid perivascular injection in order to achieve the desired effect. Studies have shown negligible evidence of tissue irritation, however, following perivascular injection of xylazine.
Intracarotid Arterial Injection Should Be Avoided. As with many compounds, including tranquilizers, immediate violent seizures followed by collapse may result from inadvertent administration into the carotid artery. Although the reaction with Rompun® (xylazine injection) is usually transient and recovery may be rapid and complete, special care should be taken to assure that the needle is in the jugular vein rather than the carotid artery.
Bradycardia and an arrhythmia in the form of incomplete atrioventricular block have been reported following Rompun® (xylazine injection) administration. Although clinically the importance of this effect is questioned,1,2,3,4 a standard dose of atropine given prior to or following Rompun® (xylazine injection) will greatly decrease the incidence.
Sedation for transport is most successful if actual transportation is begun after the full effect of the drug has been reached and the animal's stability is maintained while standing. In addition, it should be noted that animals under the influence of Rompun® (xylazine injection) can be aroused by noise or other stimuli and this may increase the risk of injury.
Cervidae: As in all ruminants, it is preferable to administer Rompun® (xylazine injection) to fasted Cervidae as a safeguard against aspiration of food material into the lungs and/or bloat during deep sedation.
Care should be taken to administer Rompun® (xylazine injection) in the heavy muscles of the croup or shoulder. Injections given subcutaneously, intraperitoneally or into fat deposits will give unpredictable results.
Intra-arterial injection should be avoided. As with many compounds, including tranquilizers, immediate violent seizures followed by collapse may result from inadvertent administration into an artery.
The animal should not be disturbed during induction or until the full effect of the drug has been reached which is usually 10 to 15 minutes following injection.
The usual time to initial effect of the drug is 2 to 5 minutes. The administrator of the drug should be fully cognizant of this interval prior to administration of drug to free ranging deer or elk, especially at night or in heavily wooded areas.
If the animal has been underdosed (faulty injection or miscalculation of weight) it is advisable to wait one hour before administering a second dose.
Adequate ventilation, especially in cages or crates, is mandatory; keep head and neck in position to insure patent air passage and to prevent aspiration of stomach contents.
During sedation animals should be prevented from assuming lateral recumbency. A sternal recumbent position is desirable.
While under the effects of Rompun® (xylazine injection) the animal should be protected from an extremely hot or cold environment.
Efforts should be made to prevent patient from rising until almost complete recovery is attained.
The transportation of Cervidae given Rompun® (xylazine injection) should be carefully monitored to prevent excessive struggling, injury or death.
Hyperthermic reactions may occur, especially if the subject is in a highly excited state when the drug is administered. Hosing the head and entire body with cold water has usually proven to be an effective deterrent.
The safety of Rompun® (xylazine injection) has not been demonstrated in pregnant Cervidae. Avoid use during the breeding season.
Cervidae should be observed closely until all of the sedative effects of Rompun® (xylazine injection) are gone.
Care should be taken at all times when administering Rompun® (xylazine injection) to Cervidae. This is due to the method of administration (usually darting), the difficulty in estimating body weights and the accepted theory that wild animals are more unpredictable in their response to sedatives and analgesics than the domesticated species.
-
WARNINGS
Human Safety: Not for human use. Keep out of reach of children.
- Strictly avoid self injection, oral intake and any contact with skin, eyes or mucosa.
- In the case of accidental contact wash exposed skin or eyes abundantly with water. If symptoms occur, seek medical advice.
- In the case of accidental oral intake or self-injection, seek the advice of a physician and show the package insert but DO NOT DRIVE.
- If pregnant women handle the product, special caution should be observed not to self-inject as uterine contractions and decreased fetal blood pressure may occur after accidental systemic exposure.
- Users with cardiovascular disease (for example, hypertension or ischemic heart disease) should take special precautions to avoid any exposure to this product.
- Caution should be exercised when handling sedated animals. Handling or any other sudden stimuli, including noise, may cause a defense reaction in an animal that appears to be heavily sedated.
Note to physician: Xylazine is an alpha2-adrenergic agonist with sedative, some analgesic and muscle relaxant properties. Symptoms after absorption may include dose-dependent respiratory depression, bradycardia, hypotension, a dry mouth, and hyperglycemia. Ventricular arrhythmias have also been reported.
The safety data sheet (SDS) contains more detailed occupational safety information. To report adverse reactions in users or to obtain a copy of the SDS for this product call 1-800-422-9874.
Animal safety: This drug should not be administered to domestic food-producing animals. Do not use in horses intended for human consumption.
In Cervidae, occasional capture-associated deaths occur. Clinical trials reveal a mortality rate of approximately 3.5% attendant with the administration of xylazine.
- HOW SUPPLIED:
- STORAGE:
-
REFERENCES:
- 1. Detweiler, D.K.: "The Diagnosis and Significance of Cardiac Arrhythmias." Progress In Equine Practice. Edited by E.J. Catcott and J.F. Smithcors. American Veterinary Publications, Inc., Santa Barbara. California and Wheaton, Illinois, (1966), 280-281.
- 2. Glazier, D.B.: "Atrioventricular Heart Block." Irish Vet. J, Vol. 12, (1958), 194-198.
- 3. Holmes, J.R., Alps, B.J.: "Observations on Partial Atrioventricular Heart Block in the Horse." Can. Vet. J., Vol. 7, No. 12, (1966), 280-290.
- 4. Smetzer, D.L., Smith, C.R., Senta, T: "Second Degree Atrioventricular Block in the Horse." Am. J. Vet. Res., Vol. 30, No. 6, (1969), 933-946.
Bayer HealthCare LLC, Animal Health Division
Shawnee Mission, Kansas 66201 U.S.A.
Made in GermanyBayer, the Bayer Cross and Rompun are registered trademarks of Bayer.
NADA 047-956, Approved by FDA
-
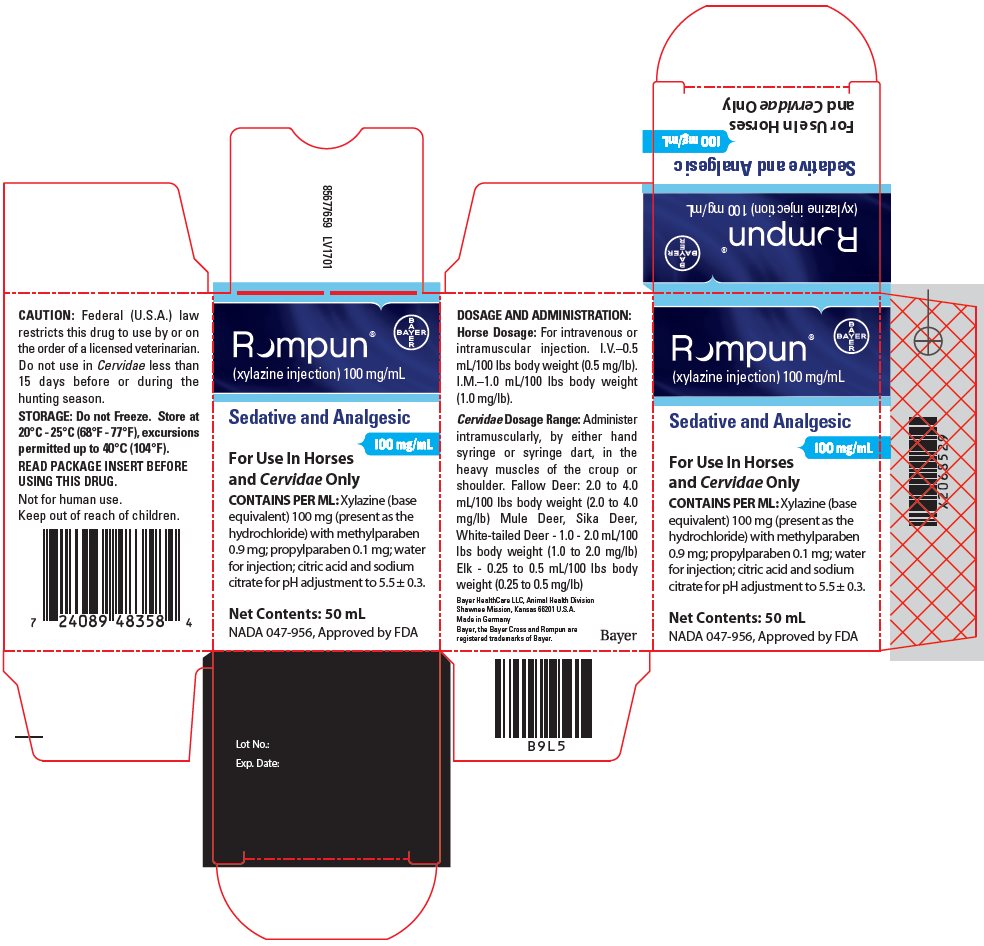
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – carton label
Rompun®
(xylazine injection) 100 mg/mLSedative and Analgesic
100 mg/mL
For Use in Horses and Cervidae Only
CONTAINS PER ML: Xylazine (base equivalent) 100 mg (present as the hydrochloride) with methylparaben 0.9 mg; propylparaben 0.1 mg; water for injection; citric acid and sodium citrate for pH adjustment to 5.5 ± 0.3.
Net Contents: 50 mL
NADA 047-956, Approved by FDA -
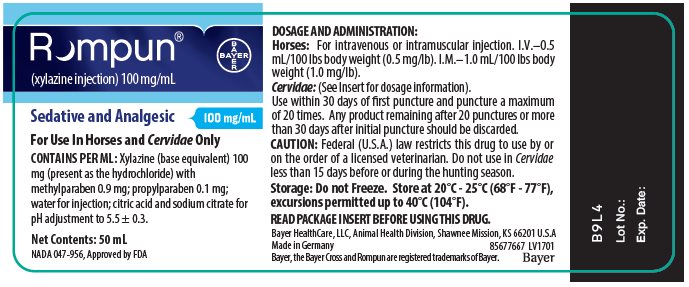
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – unit label
Rompun®
(xylazine injection) 100 mg/mLSedative and Analgesic
100 mg/mL
For Use in Horses and Cervidae Only
CONTAINS PER ML: Xylazine (base equivalent) 100 mg (present as the hydrochloride) with methylparaben 0.9 mg; propylparaben 0.1 mg; water for injection; citric acid and sodium citrate for pH adjustment to 5.5 ± 0.3.
Net Contents: 50 mL
NADA 047-956, Approved by FDA -
INGREDIENTS AND APPEARANCE
ROMPUN
xylazine injection, solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 0859-2266 Route of Administration INTRAMUSCULAR, INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength XYLAZINE (UNII: 2KFG9TP5V8) (XYLAZINE - UNII:2KFG9TP5V8) XYLAZINE 100 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0859-2266-01 1 in 1 CARTON 1 NDC: 0859-2266-02 50 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA047956 05/07/2014 Labeler - Bayer HealthCare, LLC (152266193)
Trademark Results [Rompun]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ROMPUN 86286460 4823156 Live/Registered |
Bayer Intellectual Property GmbH 2014-05-20 |
 ROMPUN 72341806 0910346 Dead/Cancelled |
FARBENFABRIKEN BAYER AKTIENGESELLSCHAFT 1969-10-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.