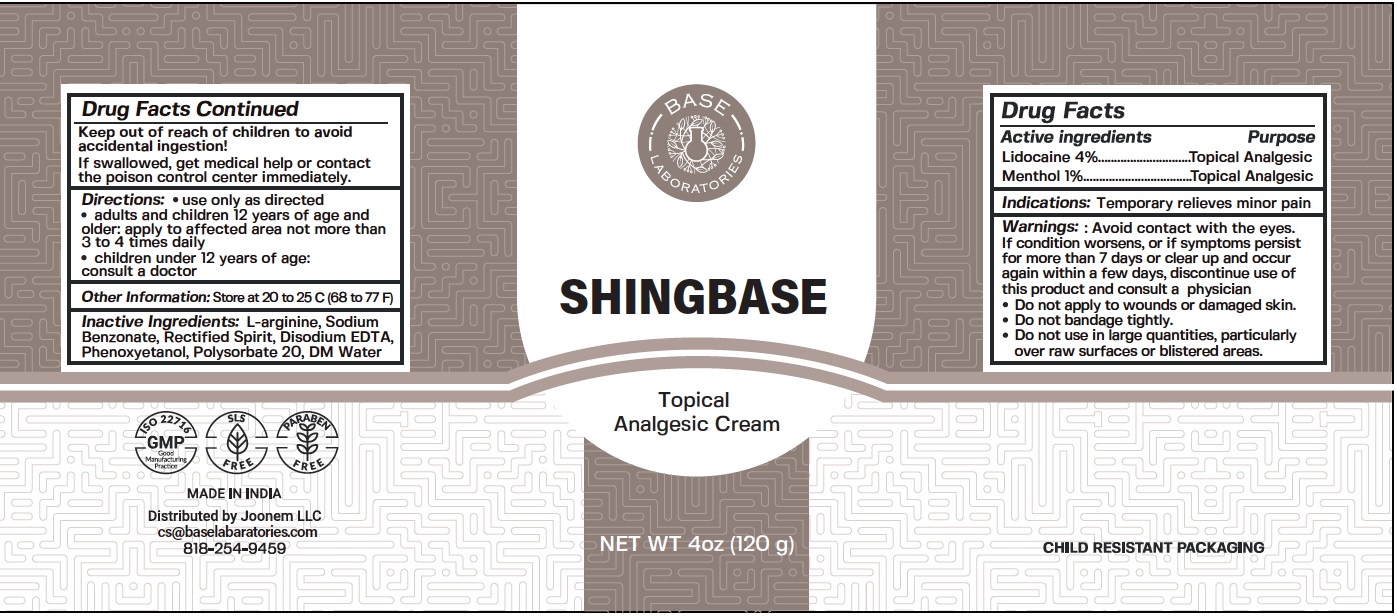
SHINGBASE Topical Analgesic Cream
SHINGBASE Topical Analgesic by
Drug Labeling and Warnings
SHINGBASE Topical Analgesic by is a Otc medication manufactured, distributed, or labeled by JOONEM LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SHINGBASE TOPICAL ANALGESIC- lidocaine, menthol creamÂ
JOONEM LLC
----------
SHINGBASE Topical Analgesic Cream
Warnings:
Avoid contact with the eyes. If condition worsens, or if symptoms persist for more than 7 days or clear up and occur again within a few days, discontinue use of this product and consult a physician
- Do not apply to wounds or damaged skin.
- Do not bandage tightly.
Directions:
- use only as directedÂ
- adults and children 12 years of age and older: apply to affected area not more than 3 to 4 times daily
- children under 12 years of age: consult a doctor
| SHINGBASE TOPICAL ANALGESICÂ
lidocaine, menthol cream |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler -Â JOONEM LLC (117633878) |
Revised: 7/2025
Â
Document Id: 3ac6260c-ca8b-030d-e063-6294a90ab5ec
Set id: afebbc7a-ed03-9b3c-e053-2a95a90a3d95
Version: 7
Effective Time: 20250725
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.