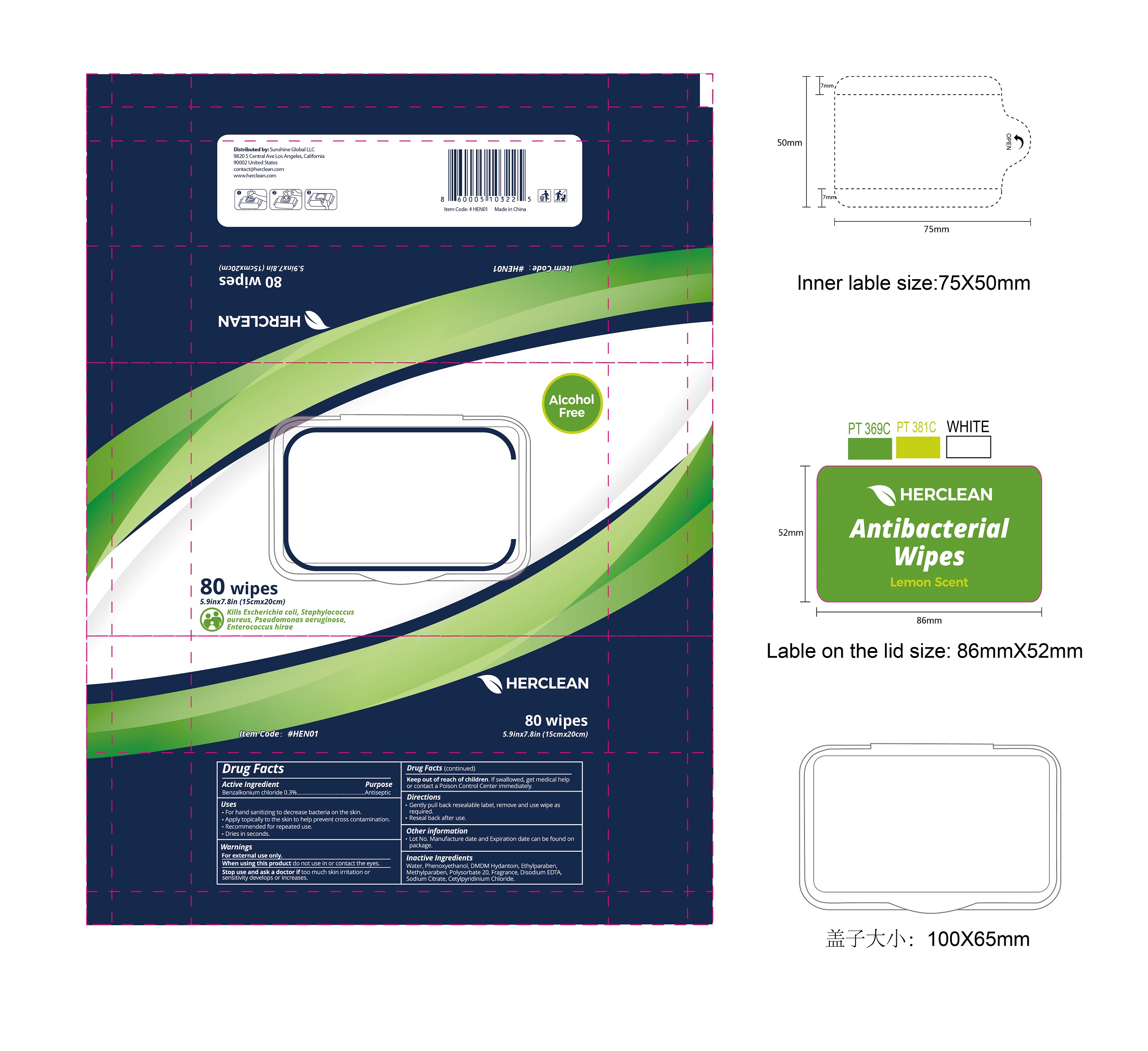
75109-517 Herclean Antibacterial Wipe 0.3% Benzalkonium Chloride
Herclean Antibacterial Wipe by
Drug Labeling and Warnings
Herclean Antibacterial Wipe by is a Otc medication manufactured, distributed, or labeled by Kangna (Zhejiang) Medical Supplies Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
HERCLEAN ANTIBACTERIAL WIPE- benzalkonium chloride cloth
Kangna (Zhejiang) Medical Supplies Co., Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
75109-517 Herclean Antibacterial Wipe 0.3% Benzalkonium Chloride
USE
●For hand sanitizing to decrease bacteria on the skin.
●Apply topically to the skin to help prevent cross contamination.
●Not recommended for repeated use.
●Dries in seconds.
Warning
For external use only.
When using this product do not use in or contact the eyes.
Stop use and ask a doctor if too much skin irritation or
sensitivity develops or increases.
Directions
.Gently pull back resealable label, remove and use wipe as required.
.Reseal back after use.
Other information
.Lot No. Manufacture date and Expiration date can be found on package.
| HERCLEAN ANTIBACTERIAL WIPE
benzalkonium chloride cloth |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Kangna (Zhejiang) Medical Supplies Co., Ltd. (554530173) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Kangna (Zhejiang) Medical Supplies Co., Ltd. | 554530173 | manufacture(75109-517) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.