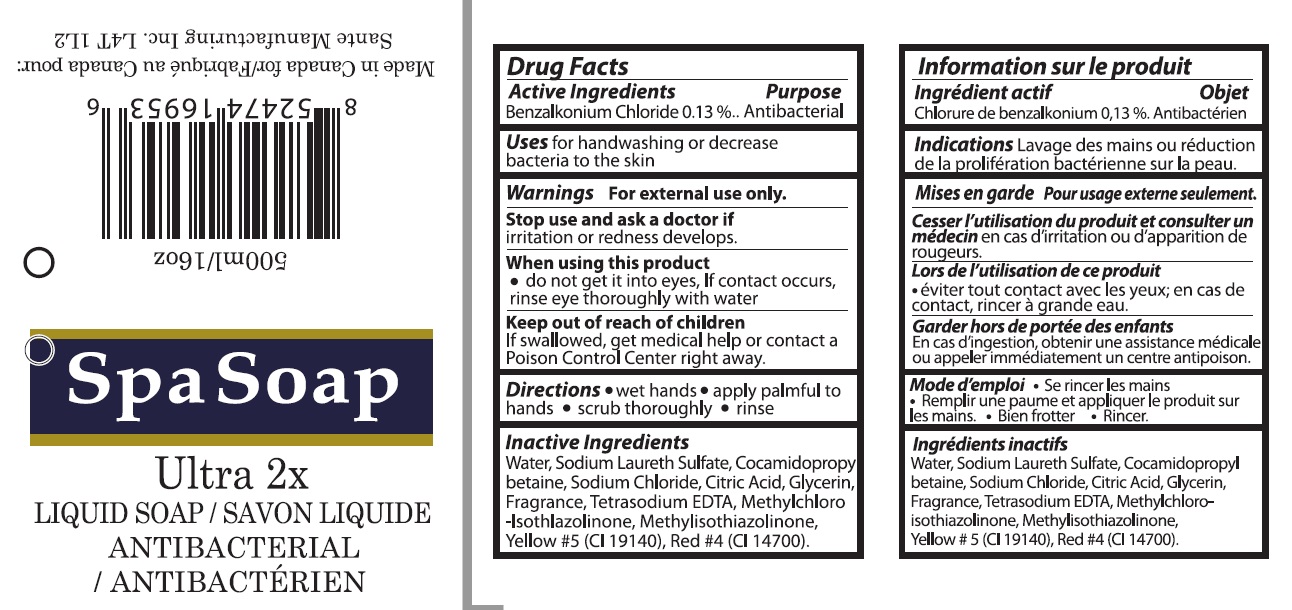
Spa Soap Ultra 2X Liquld Soap
Antibacterial by
Drug Labeling and Warnings
Antibacterial by is a Otc medication manufactured, distributed, or labeled by Total Body Care Inc, Sante Manufcaturing Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ANTIBACTERIAL
UTRA 2X- benzalkonium chloride liquid
Total Body Care Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Spa Soap Ultra 2X Liquld Soap
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away
| ANTIBACTERIAL
UTRA 2X
benzalkonium chloride liquid |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Total Body Care Inc (250919615) |
| Registrant - Sante Manufcaturing Inc (242048747) |
Revised: 1/2022
Document Id: d681d906-77f1-9bb8-e053-2a95a90af093
Set id: b00026ba-55ed-f298-e053-2995a90a3937
Version: 3
Effective Time: 20220126
Trademark Results [Antibacterial]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ANTIBACTERIAL 79333776 not registered Live/Pending |
BIC Graphic Europe SA 2021-11-26 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.