Rite Aid Cold Sore Treatment by Rite Aid / Ranir LLC BPC 407 - Rite Aid
Rite Aid Cold Sore Treatment by
Drug Labeling and Warnings
Rite Aid Cold Sore Treatment by is a Otc medication manufactured, distributed, or labeled by Rite Aid, Ranir LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
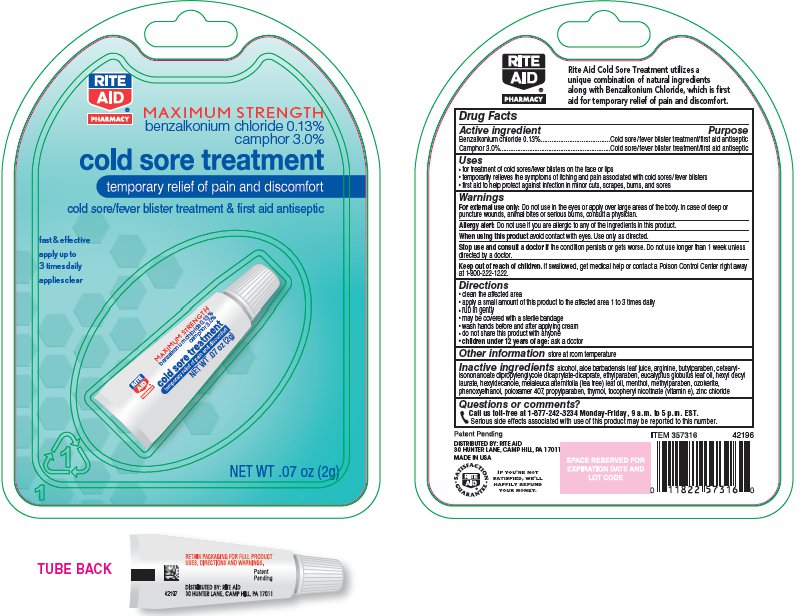
RITE AID COLD SORE TREATMENT- camphor cream
Rite Aid
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
BPC 407 - Rite Aid
Uses
- for treatment of cold sores/fever blisters on the face or lips
- temporarily relieves the symptoms of itching and pain associated with cold sores/fever blisters
- first aid to help protect against infection in minor cuts, scrapes, burns, and First aid to help protect against infection in minor cuts, scrapes, burns, and sores
Warnings
- For external use only: Do not use in the eyes or apply over large areas of the body. In case of deep or puncture wounds, animal bites or serious burns, consult a physician.
- Allergy Alert: Do not use if you are allergic to any of the ingredients in this product.
- When using this product avoid contact with eyes. Use only as directed.
- Stop use and consult a doctor if the condition persists or gets worse. Do not use longer than 1 week unless directed by a doctor.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away at 1-800-222-1222.
Directions
- clean the affected area
- apply a small amount of this product to the affected area 1 to 3 times daily
- rub in gently
- wash hands before and after applying the product
- do not share this product with anyone
- children under 12 years of age: ask a doctor
Inactive Ingredients
alcohol, aloe barbadensis leaf juice, arginine, butylparaben, cetearyl isononanoate, dipropylene glycol dicaprylate/dicaprate, ethylparaben, eucalyptus globulus leaf oil, hexyl decyl laurate, hexyldecanole, melaleuca alternifolia (tea tree) leaf oil, menthol, methylparaben, ozokerite, phenoxyethanol, poloxamer 407, propylparaben, thymol, tocopheryl nicotinate (vitamin e), zinc chloride
| RITE AID COLD SORE TREATMENT
camphor cream |
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Rite Aid (014578892) |
| Registrant - Ranir LLC (364567615) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ranir LLC | 364567615 | repack(11822-4070) , relabel(11822-4070) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.