THERABENZAPRINE-60- cyclobenzaprine hydrochloride, .gamma.-aminobutyric acid kit
Therabenzaprine-60 by
Drug Labeling and Warnings
Therabenzaprine-60 by is a Prescription medication manufactured, distributed, or labeled by Physician Therapeutics LLC, Vintage Pharmaceuticals, Targeted Medical Pharma Inc., H.J. Harkins Company, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
DESCRIPTION
Cyclobenzaprine hydrochloride is a white, crystalline tricyclic amine salt with the empirical formula C20H21N HCl and a molecular weight of 311.9. It has a melting point of 217°C, and a pKa of 8.47 at 25°C. It is freely soluble in water and alcohol, sparingly soluble in isopropanol, and insoluble in hydrocarbon solvents. If aqueous solutions are made alkaline, the free base separates.
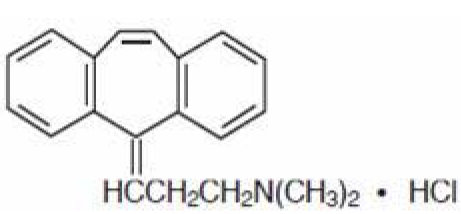
Cyclobenzaprine HCl is designated chemically as 3-(5H-dibenzo[a,d]cyclohepten-5-ylidene)-N,N-dimethyl-1-propanamine hydrochloride, and has the following structural formula:
Cyclobenzaprine Hydrochloride Tablets, USP are supplied as 5 mg and 10 mg tablets for oral administration.
Each tablet contains the following inactive ingredients: croscarmellose sodium, FD C Yellow #6, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, and titanium dioxide; 5 mg tablets also contain FD C Red #40 and 10 mg tablets contain D C Yellow #10 and polysorbate.
-
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGY
Cyclobenzaprine HCl relieves skeletal muscle spasm of local origin without interfering with muscle function. It is ineffective in muscle spasm due to central nervous system disease.
Cyclobenzaprine reduced or abolished skeletal muscle hyperactivity in several animal models. Animal studies indicate that cyclobenzaprine does not act at the neuromuscular junction or directly on skeletal muscle. Such studies show that cyclobenzaprine acts primarily within the central nervous system at brain stem as opposed to spinal cord levels, although its action on the latter may contribute to its overall skeletal muscle relaxant activity. Evidence suggests that the net effect of cyclobenzaprine is a reduction of tonic somatic motor activity, influencing both gamma (γ) and alpha (α) motor systems.
Pharmacological studies in animals showed a similarity between the effects of cyclobenzaprine and the structurally related tricyclic antidepressants, including reserpine antagonism, norepinephrine potentiation, potent peripheral and central anticholinergic effects, and sedation. Cyclobenzaprine caused slight to moderate increase in heart rate in animals.
-
PHARMACOKINETICS
Pharmacokinetics
Estimates of mean oral bioavailability of cyclobenzaprine range from 33% to 55%. Cyclobenzaprine exhibits linear pharmacokinetics over the dose range 2.5 mg to 10 mg, and is subject to enterohepatic circulation. It is highly bound to plasma proteins. Drug accumulates when dosed three times a day, reaching steady state within 3-4 days at plasma concentrations about four-fold higher than after a single dose. At steady state in healthy subjects receiving 10 mg t.i.d. (n=18), peak plasma concentration was 25.9 ng/mL (range, 12.8-46.1 ng/mL), and area under the concentration-time (AUC) curve over an 8-hour dosing interval was 177 nghr/mL (range, 80-319 nghr/mL).
Cyclobenzaprine is extensively metabolized, and is excreted primarily as glucuronides via the kidney. Cytochromes P-450 3A4, 1A2, and, to a lesser extent, 2D6, mediate N-demethylation, one of the oxidative pathways for cyclobenzaprine. Cyclobenzaprine is eliminated quite slowly, with an effective half-life of 18 hours (range 8-37 hours; n=18); plasma clearance is 0.7 L/min.
The plasma concentration of cyclobenzaprine is generally higher in the elderly and in patients with hepatic impairment (see PRECAUTIONS, Use in the Elderly and PRECAUTIONS, Impaired Hepatic Function).
ElderlyIn a pharmacokinetic study in elderly individuals (≥65 yrs old), mean (n=10) steady state cyclobenzaprine AUC values were approximately 1.7-fold (171.0 nghr/mL, range 96.1-255.3) higher than those seen in a group of eighteen younger adults (101.4 nghr/mL, range 36.1-182.9) from another study. Elderly male subjects had the highest observed mean increase, approximately 2.4-fold (198.3 nghr/mL, range 155.6-255.3 versus 83.2 nghr/mL, range 41.1-142.5 for younger males) while levels in elderly females were increased to a much lesser extent, approximately 1.2-fold (143.8 nghr/mL, range 96.1-196.3 versus 115.9 nghr/mL, range 36.1-182.9 for younger females).
In light of these findings, therapy with cyclobenzaprine hydrochloride tablets in the elderly should be initiated with a 5 mg dose and titrated slowly upward.
Hepatic ImpairmentIn a pharmacokinetic study of sixteen subjects with hepatic impairment (15 mild, 1 moderate per Child-Pugh score), both AUC and Cmax were approximately double the values seen in the healthy control group. Based on the findings, cyclobenzaprine hydrochloride tablets should be used with caution in subjects with mild hepatic impairment starting with the 5 mg dose and titrating slowly upward. Due to the lack of data in subjects with more severe hepatic insufficiency, the use of cyclobenzaprine hydrochloride tablets in subjects with moderate to severe impairment is not recommended.
No significant effect on plasma levels or bioavailability of cyclobenzaprine hydrochloride tablets or aspirin was noted when single or multiple doses of the two drugs were administered concomitantly. Concomitant administration of cyclobenzaprine hydrochloride tablets and naproxen or diflunisal was well tolerated with no reported unexpected adverse effects. However combination therapy of cyclobenzaprine hydrochloride tablets with naproxen was associated with more side effects than therapy with naproxen alone, primarily in the form of drowsiness. No well-controlled studies have been performed to indicate that cyclobenzaprine hydrochloride tablets enhance the clinical effect of aspirin or other analgesics, or whether analgesics enhance the clinical effect of cyclobenzaprine hydrochloride tablets in acute musculoskeletal conditions.
Clinical StudiesEight double-blind controlled clinical studies were performed in 642 patients comparing cyclobenzaprine hydrochloride 10 mg, diazepam, and placebo. Muscle spasm, local pain and tenderness, limitation of motion, and restriction in activities of daily living were evaluated. In three of these studies there was a significantly greater improvement with cyclobenzaprine than with diazepam, while in the other studies the improvement following both treatments was comparable.
Although the frequency and severity of adverse reactions observed in patients treated with cyclobenzaprine were comparable to those observed in patients treated with diazepam, dry mouth was observed more frequently in patients treated with cyclobenzaprine and dizziness more frequently in those treated with diazepam. The incidence of drowsiness, the most frequent adverse reaction, was similar with both drugs.
The efficacy of cyclobenzaprine hydrochloride tablets 5 mg was demonstrated in two seven-day, double-blind, controlled clinical trials enrolling 1405 patients. One study compared cyclobenzaprine hydrochloride tablets 5 and 10 mg t.i.d. to placebo; and a second study compared cyclobenzaprine hydrochloride tablets 5 and 2.5 mg t.i.d. to placebo. Primary endpoints for both trials were determined by patient-generated data and included global impression of change, medication helpfulness, and relief from starting backache. Each endpoint consisted of a score on a 5-point rating scale (from 0 or worst outcome to 4 or best outcome). Secondary endpoints included a physician's evaluation of the presence and extent of palpable muscle spasm.
Comparisons of cyclobenzaprine hydrochloride tablets 5 mg and placebo groups in both trials established the statistically significant superiority of the 5 mg dose for all three primary endpoints at day 8 and, in the study comparing 5 and 10 mg, at day 3 or 4 as well. A similar effect was observed with cyclobenzaprine hydrochloride tablets 10 mg (all endpoints). Physician-assessed secondary endpoints also showed that cyclobenzaprine hydrochloride tablets 5 mg was associated with a greater reduction in palpable muscle spasm than placebo.
Analysis of the data from controlled studies shows that cyclobenzaprine produces clinical improvement whether or not sedation occurs.
Surveillance ProgramA post-marketing surveillance program was carried out in 7607 patients with acute musculoskeletal disorders, and included 297 patients treated with cyclobenzaprine hydrochloride tablets 10 mg for 30 days or longer. The overall effectiveness of cyclobenzaprine was similar to that observed in the double-blind controlled studies; the overall incidence of adverse effects was less (see ADVERSE REACTIONS).
-
INDICATIONS & USAGE
INDICATIONS AND USAGE
Cyclobenzaprine hydrochloride tablets are indicated as an adjunct to rest and physical therapy for relief of muscle spasm associated with acute, painful musculoskeletal conditions.
Improvement is manifested by relief of muscle spasm and its associated signs and symptoms, namely, pain, tenderness, limitation of motion, and restriction in activities of daily living.
Cyclobenzaprine hydrochloride tablets should be used only for short periods (up to two or three weeks) because adequate evidence of effectiveness for more prolonged use is not available and because muscle spasm associated with acute, painful musculoskeletal conditions is generally of short duration and specific therapy for longer periods is seldom warranted.
Cyclobenzaprine hydrochloride tablets have not been found effective in the treatment of spasticity associated with cerebral or spinal cord disease, or in children with cerebral palsy.
-
CONTRAINDICATIONS
CONTRAINDICATIONS
Hypersensitivity to any component of this product.
Concomitant use of monoamine oxidase (MAO) inhibitors or within 14 days after their discontinuation. Hyperpyretic crisis seizures, and deaths have occurred in patients receiving cyclobenzaprine (or structurally similar tricyclic antidepressants) concomitantly with MAO inhibitor drugs.
Acute recovery phase of myocardial infarction, and patients with arrhythmias, heart block or conduction disturbances, or congestive heart failure.
Hyperthyroidism.
-
WARNINGS
WARNINGS
Cyclobenzaprine is closely related to the tricyclic antidepressants, e.g., amitriptyline and imipramine. In short term studies for indications other than muscle spasm associated with acute musculoskeletal conditions, and usually at doses somewhat greater than those recommended for skeletal muscle spasm, some of the more serious central nervous system reactions noted with the tricyclic antidepressants have occurred (see WARNINGS, below, and ADVERSE REACTIONS).
Tricyclic antidepressants have been reported to produce arrhythmias, sinus tachycardia, prolongation of the conduction time leading to myocardial infarction and stroke.
Cyclobenzaprine may enhance the effects of alcohol, barbiturates, and other CNS depressants.
-
PRECAUTIONS
PRECAUTIONSGeneral
Because of its atropine-like action, cyclobenzaprine hydrochloride should be used with caution in patients with a history of urinary retention, angle-closure glaucoma, increased intraocular pressure, and in patients taking anticholinergic medication.
Impaired Hepatic FunctionThe plasma concentration of cyclobenzaprine is increased in patients with hepatic impairment (see CLINICAL PHARMACOLOGY, Pharmacokinetics, Hepatic Impairment).
These patients are generally more susceptible to drugs with potentially sedating effects, including cyclobenzaprine. Cyclobenzaprine hydrochloride tablets should be used with caution in subjects with mild hepatic impairment starting with a 5 mg dose and titrating slowly upward. Due to the lack of data in subjects with more severe hepatic insufficiency, the use of cyclobenzaprine hydrochloride tablets in subjects with moderate to severe impairment is not recommended.
-
INFORMATION FOR PATIENTS
Information for Patients
Cyclobenzaprine hydrochloride tablets, especially when used with alcohol or other CNS depressants, may impair mental and/or physical abilities required for performance of hazardous tasks, such as operating machinery or driving a motor vehicle. In the elderly, the frequency and severity of adverse events associated with the use of cyclobenzaprine, with or without concomitant medications, is increased. In elderly patients, cyclobenzaprine hydrochloride tablets should be initiated with a 5 mg dose and titrated slowly upward.
-
DRUG INTERACTIONS
Drug Interactions
Cyclobenzaprine may have life-threatening interactions with MAO inhibitors (see CONTRAINDICATIONS).
Cyclobenzaprine may enhance the effects of alcohol, barbiturates, and other CNS depressants.
Tricyclic antidepressants may block the antihypertensive action of guanethidine and similarly acting compounds.
Tricyclic antidepressants may enhance the seizure risk in patients taking tramadol.
-
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
Carcinogenesis, Mutagenesis, Impairment of Fertility
In rats treated with cyclobenzaprine hydrochloride for up to 67 weeks at doses of approximately 5 to 40 times the maximum recommended human dose, pale, sometimes enlarged, livers were noted and there was a dose-related hepatocyte vacuolation with lipidosis. In the higher dose groups this microscopic change was seen after 26 weeks and even earlier in rats which died prior to 26 weeks; at lower doses, the change was not seen until after 26 weeks.
Cyclobenzaprine did not affect the onset, incidence or distribution of neoplasia in an 81-week study in the mouse or in a 105-week study in the rat.
At oral doses of up to 10 times the human dose, cyclobenzaprine did not adversely affect the reproductive performance or fertility of male or female rats. Cyclobenzaprine did not demonstrate mutagenic activity in the male mouse at dose levels of up to 20 times the human dose.
-
PREGNANCY
PregnancyPregnancy Category B:
Reproduction studies have been performed in rats, mice and rabbits at doses up to 20 times the human dose, and have revealed no evidence of impaired fertility or harm to the fetus due to cyclobenzaprine. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
-
NURSING MOTHERS
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because cyclobenzaprine is closely related to the tricyclic antidepressants, some of which are known to be excreted in human milk, caution should be exercised when cyclobenzaprine hydrochloride is administered to a nursing woman.
-
PEDIATRIC USE
Pediatric Use
Safety and effectiveness of cyclobenzaprine hydrochloride in pediatric patients below 15 years of age have not been established.
Use in the ElderlyThe plasma concentration of cyclobenzaprine is increased in the elderly (see CLINICAL PHARMACOLOGY, Pharmacokinetics, Elderly). The elderly may also be more at risk for CNS adverse events such as hallucinations and confusion, cardiac events resulting in falls or other sequelae, drug-drug and drug-disease interactions. For these reasons, in the elderly, cyclobenzaprine should be used only if clearly needed. In such patients cyclobenzaprine should be initiated with a 5 mg dose and titrated slowly upward.
-
ADVERSE REACTIONS
ADVERSE REACTIONS Incidence of most common adverse reactions in the 2 double-blind, placebo-controlled 5 mg studies (incidence of greater than 3% on cyclobenzaprine hydrochloride 5 mg):
Cyclobenzaprine
HCl 5 mg
N=464
Cyclobenzaprine
HCl 10 mg
N=249
Placebo
N=469
Drowsiness
29%
38%
10%
Dry Mouth
21%
32%
7%
Fatigue
6%
6%
3%
Headache
5%
5%
8%
Adverse reactions which were reported in 1% to 3% of the patients were: abdominal pain, acid regurgitation, constipation, diarrhea, dizziness, nausea, irritability, mental acuity decreased, nervousness, upper respiratory infection, and pharyngitis.
The following list of adverse reactions is based on the experience in 473 patients treated with cyclobenzaprine hydrochloride tablets 10 mg in additional controlled clinical studies, 7607 patients in the post-marketing surveillance program, and reports received since the drug was marketed. The overall incidence of adverse reactions among patients in the surveillance program was less than the incidence in the controlled clinical studies.
The adverse reactions reported most frequently with cyclobenzaprine hydrochloride were drowsiness, dry mouth and dizziness. The incidence of these common adverse reactions was lower in the surveillance program than in the controlled clinical studies:
Note: Cyclobenzaprine hydrochloride tablets 10 mg data are from one clinical trial. Cyclobenzaprine hydrochloride tablets 5 mg and placebo data are from two studies.
Clinical Studies With
Cyclobenzaprine HCl 10 mg
Surveillance Progam
With Cyclobenzaprine HCl 10 mg
Drowsiness
39%
16%
Dry Mouth
27%
7%
Dizziness
11%
3%
Among the less frequent adverse reactions, there was no appreciable difference in incidence in controlled clinical studies or in the surveillance program. Adverse reactions which were reported in 1% to 3% of the patients were: fatigue/tiredness, asthenia, nausea, constipation, dyspepsia, unpleasant taste, blurred vision, headache, nervousness, and confusion.
The following adverse reactions have been reported in post-marketing experience or with an incidence of less than 1% of patients in clinical trials with the 10 mg tablet:
Body as a Whole: Syncope; malaise.
Cardiovascular: Tachycardia; arrhythmia; vasodilatation; palpitation; hypotension.
Digestive: Vomiting; anorexia; diarrhea; gastrointestinal pain; gastritis; thirst; flatulence; edema of the tongue; abnormal liver function and rare reports of hepatitis, jaundice and cholestasis.
Hypersensitivity: Anaphylaxis; angioedema; pruritus; facial edema; urticaria; rash.
Musculoskeletal: Local weakness.
Nervous System and Psychiatric: Seizures, ataxia; vertigo; dysarthria; tremors; hypertonia; convulsions; muscle twitching; disorientation; insomnia; depressed mood; abnormal sensations; anxiety; agitation; psychosis, abnormal thinking and dreaming; hallucinations; excitement; paresthesia; diplopia.
Skin: Sweating.
Special Senses: Ageusia; tinnitus.
Urogenital: Urinary frequency and/or retention.Causal Relationship Unknown
Other reactions, reported rarely for cyclobenzaprine hydrochloride under circumstances where a causal relationship could not be established or reported for other tricyclic drugs, are listed to serve as alerting information to physicians:
Body as a Whole: Chest pain; edema.
Cardiovascular: Hypertension; myocardial infarction; heart block; stroke.
Digestive: Paralytic ileus; tongue discoloration; stomatitis; parotid swelling.
Endocrine: Inappropriate ADH syndrome.
Hematic and Lymphatic: Purpura; bone marrow depression; leukopenia; eosinophilia; thrombocytopenia.
Metabolic, Nutritional and Immune: Elevation and lowering of blood sugar levels; weight gain or loss.
Musculoskeletal: Myalgia.
Nervous System and Psychiatric: Decreased or increased libido; abnormal gait; delusions; aggressive behavior; paranoia; peripheral neuropathy; Bell's palsy; alteration in EEG patterns; extrapyramidal symptoms.
Respiratory: Dyspnea.
Skin: Photosensitization; alopecia.
Urogenital: Impaired urination; dilatation of urinary tract; impotence; testicular swelling; gynecomastia; breast enlargement; galactorrhea.
-
DRUG ABUSE AND DEPENDENCE
DRUG ABUSE AND DEPENDENCE
Pharmacologic similarities among the tricyclic drugs require that certain withdrawal symptoms be considered when cyclobenzaprine hydrochloride is administered, even though they have not been reported to occur with this drug. Abrupt cessation of treatment after prolonged administration rarely may produce nausea, headache, and malaise. These are not indicative of addiction.
-
OVERDOSAGE
OVERDOSAGE
Although rare, deaths may occur from overdosage with cyclobenzaprine hydrochloride. Multiple drug ingestion (including alcohol) is common in deliberate cyclobenzaprine overdose. As management of overdose is complex and changing, it is recommended that the physician contact a poison control center for current information on treatment. Signs and symptoms of toxicity may develop rapidly after cyclobenzaprine overdose; therefore, hospital monitoring is required as soon as possible. The acute oral LD 50 of cyclobenzaprine hydrochloride is approximately 338 and 425 mg/kg in mice and rats, respectively.
MANIFESTATIONS
The most common effects associated with cyclobenzaprine overdose are drowsiness and tachycardia. Less frequent manifestations include tremor, agitation, coma, ataxia, hypertension, slurred speech, confusion, dizziness, nausea, vomiting, and hallucinations. Rare but potentially critical manifestations of overdose are cardiac arrest, chest pain, cardiac dysrhythmias, severe hypotension, seizures, and neuroleptic malignant syndrome. Changes in the electrocardiogram, particularly in QRS axis or width, are clinically significant indicators of cyclobenzaprine toxicity.
Other potential effects of overdosage include any of the symptoms listed under ADVERSE REACTIONS.
MANAGEMENT
General
As management of overdose is complex and changing, it is recommended that the physician contact a poison control center for current information on treatment.
In order to protect against the rare but potentially critical manifestations described above, obtain an ECG and immediately initiate cardiac monitoring. Protect the patient's airway, establish an intravenous line and initiate gastric decontamination. Observation with cardiac monitoring and observation for signs of CNS or respiratory depression, hypotension, cardiac dysrhythmias and/or conduction blocks, and seizures is necessary. If signs of toxicity occur at any time during this period, extended monitoring is required. Monitoring of plasma drug levels should not guide management of the patient. Dialysis is probably of no value because of low plasma concentrations of the drug.
Gastrointestinal Decontamination
All patients suspected of an overdose with cyclobenzaprine hydrochloride should receive gastrointestinal decontamination. This should include large volume gastric lavage followed by activated charcoal. If consciousness is impaired, the airway should be secured prior to lavage and emesis is contraindicated.
Cardiovascular
A maximal limb-lead QRS duration of less than equal than 0.10 seconds may be the best indication of the severity of the overdose. Serum alkalinization, to a pH of 7.45 to 7.55, using intravenous sodium bicarbonate and hyperventilation (as needed), should be instituted for patients with dysrhythmias and/or QRS widening. A pH >7.60 or a pCO2 less than 20 mmHg is undesirable. Dysrhythmias unresponsive to sodium bicarbonate therapy/hyperventilation may respond to lidocaine, bretylium or phenytoin. Type 1A and 1C antiarrhythmics are generally contraindicated (e.g., quinidine, disopyramide, and procainamide).
CNS
In patients with CNS depression, early intubation is advised because of the potential for abrupt deterioration. Seizures should be controlled with benzodiazepines or, if these are ineffective, other anticonvulsants (e.g., phenobarbital, phenytoin). Physostigmine is not recommended except to treat life-threatening symptoms that have been unresponsive to other therapies, and then only in close consultation with a poison control center.
PSYCHIATRIC FOLLOW-UP
Since overdosage is often deliberate, patients may attempt suicide by other means during the recovery phase. Psychiatric referral may be appropriate.
PEDIATRIC MANAGEMENT
The principles of management of child and adult overdosages are similar. It is strongly recommended that the physician contact the local poison control center for specific pediatric treatment. -
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION
For most patients, the recommended dose of cyclobenzaprine hydrochloride tablets is 5 mg three times a day. Based on individual patient response, the dose may be increased to 10 mg three times a day. Use of cyclobenzaprine hydrochloride tablets for periods longer than two or three weeks is not recommended (see INDICATIONS AND USAGE).
Less frequent dosing should be considered for hepatically impaired or elderly patients (see PRECAUTIONS, Impaired Hepatic Function, and Use in the Elderly).
-
HOW SUPPLIED
HOW SUPPLIED
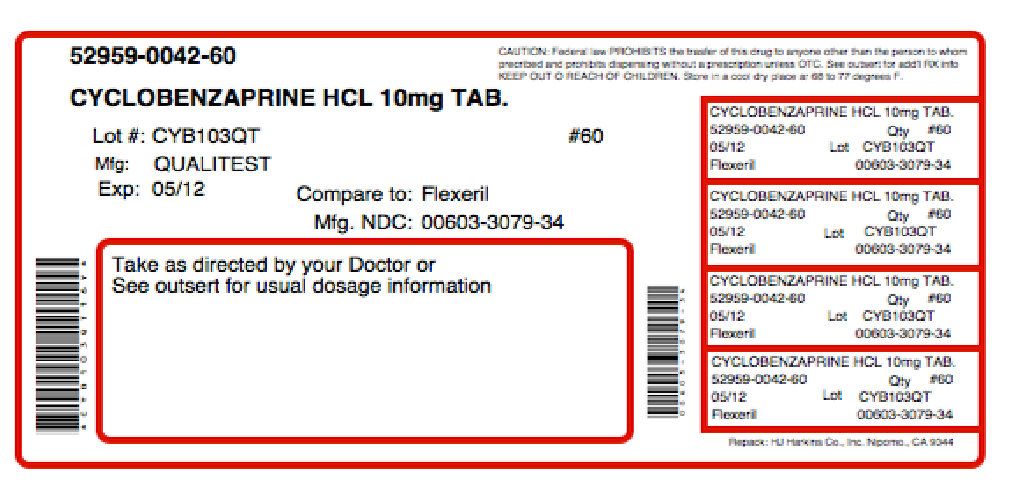
Cyclobenzaprine Hydrochloride Tablets, USP 5 mg round, orange film-coated tablets, debossed "2631" on one side and debossed "V" on the reverse side, supplied in bottles of 10, 100, 500 and 1000.
Cyclobenzaprine Hydrochloride Tablets, USP 10 mg round, yellow film-coated tablets, debossed "2632" on one side and debossed "V" on the reverse side, supplied in bottles of 10, 90, 100, 180, 270, 500, 1000 and 5000.
Manufactured for:
QUALITEST PHARMACEUTICALS
Huntsville, AL 35811
8181950
R6/11-R2
- STORAGE AND HANDLING
- KEEP OUT OF REACH OF CHILDREN
- PRINCIPAL DISPLAY PANEL
-
SPL UNCLASSIFIED SECTION
Theramine (U.S. patent pending) capsules by oral administration. A specially formulated Medical Food product, consisting of a proprietary blend of amino acids and polyphenol ingredients in specific proportions, for the dietary management of the metabolic processes associated with pain disorders and inflammatory conditions. (PD) (IC). Must be administered under physician supervision.
Medical Foods
Medical Food products are often used in hospitals (e.g., for burn victims or kidney dialysis patients) and outside of a hospital setting under a physician’s care for the dietary management of diseases in patients with particular, unique or distinctive medical or metabolic needs due to their disease or condition. Congress defined "Medical Food" in the Orphan Drug Act and Amendments of 1988 as "a food which is formulated to be consumed or administered enterally [or orally] under the supervision of a physician and which is intended for the specific dietary management of a disease or condition for which distinctive nutritional requirements, based on recognized scientific principles, are established by medical evaluation." Medical Foods are complex formulated products, requiring sophisticated and exacting technology, and that are used only for a patient receiving active and ongoing medical supervision wherein the patient requires medical care on a recurring basis for, among other things, instructions on the use of the Medical Food. Theramine has been developed, manufactured, and labeled in accordance with both the statutory definition of a Medical Food and FDA’s regulatory labeling guidelines. Theramine must be used while the patient is under the ongoing care of a physician.
PAIN DISORDERS (PD) INFLAMMATORY CONDITIONS (IC)
PD and IC as a Metabolic Deficiency Disease
A critical component of the definition of a Medical Food is that the product must address the distinct nutritional requirements of a particular disease or condition. FDA scientists have proposed a physiologic definition of distinctive nutritional requirements as follows: “the dietary management of patients with specific diseases requires, in some instances, the ability to meet nutritional requirements that differ substantially from the needs of healthy persons. For example, in establishing the recommended dietary allowances for general, healthy population, the Food and Nutrition Board of the Institute of Medicine National Academy of Sciences, recognized that different or distinctive physiologic requirements may exist for certain persons with "special nutritional needs arising from metabolic disorders, chronic diseases, injuries, premature birth, other medical conditions and drug therapies. Thus, the distinctive nutritional needs associated with a disease reflects the total amount needed by a healthy person to support life or maintain homeostasis, adjusted for the distinctive changes in the nutritional needs of the patient as a result of the effects of the disease process on absorption, metabolism and excretion.” It was also proposed that in patients with certain disease states who respond to nutritional therapies, a physiologic deficiency of the nutrient is assumed to exist. For example, if a patient with a pain disorder responds to a tryptophan formulation by decreasing perceived pain, then a deficiency of tryptophan is assumed to exist. Patients with pain disorders and inflammatory conditions are known to have increased nutritional requirements for tryptophan, choline, arginine, GABA, flavonoids, and certain antioxidants. These nutritional requirements are such that they cannot be achieved by the modification of the normal diet alone, or by supplementing the diet.
Patients with pain disorders and inflammatory conditions frequently exhibit reduced plasma levels of tryptophan and GABA, and have been shown to respond to oral administration of GABA, arginine, tryptophan, or a 5-hydroxytryptophan formulation. Research has shown that tryptophan, arginine or GABA reduced diets result in a fall of circulating tryptophan, arginine, and/or GABA. Patients with pain disorders frequently exhibit activation of the degradation pathways that increases the turnover rate of GABA, arginine and/or tryptophan leading to a reduced level of production of serotonin, GABA or nitric oxide for a given precursor blood level. Research has also shown that a genetic predisposition to accelerated degradation can lead to increased precursor requirements in certain patients with pain disorders and inflammatory conditions.
Choline is required to fully potentiate acetylcholine synthesis by brain neurons. A deficiency of choline leads to reduced acetylcholine production by the neurons. Provision of tryptophan, arginine, GABA, choline and flavonoids with antioxidants, in specific proportions can restore the production of beneficial serotonin, nitric oxide, and acetylcholine, thereby reducing the perception of pain and reducing inflammation. L-Histidine is known to produce brain histamine that stimulates production of ACTH, producing cortisol to reduce inflammation.
-
DESCRIPTION
PRODUCT DESCRIPTION
Primary Ingredients
Theramine consists of a proprietary formulation of Gamma Aminobutyric Acid, Choline Bitartrate, Whey Protein Hydrolysate, L-Arginine, L-Histidine, L-Glutamine, Theobromine, Griffonia See, Grape Seed, L-Serine, and Cinnamon in specific proportions. These ingredients fall into the classification of Generally Recognized as Safe (GRAS) as defined by the Food and Drug Administration (FDA) (Sections 201(s) and 409 of the Federal Food, Drug, and Cosmetic Act). A GRAS substance is distinguished from a food additive on the basis of the common knowledge about the safety of the substance for its intended use. The standard for an ingredient to achieve GRAS status requires not only technical demonstration of non-toxicity and safety, but also general recognition of safety through widespread usage and agreement of that safety by experts in the field. Many ingredients have been determined by the FDA to be GRAS, and are listed as such by regulation, in Volume 21 Code of Federal Regulations (CFR) Sections 182, 184, and 186.
Amino Acids
Amino Acids are the building blocks of protein and are GRAS listed as they have been safely ingested by humans for thousands of years. The formulations of the amino acids in Theramine are equivalent to those found in the usual human diet. Patients with pain disorders may require an increased amount of certain amino acids that cannot be obtained from normal diet alone. Tryptophan, for example, is an obligatory amino acid. The body cannot make tryptophan and must obtain tryptophan from the diet. Tryptophan is needed to produce serotonin. Serotonin is required to reduce pain. Patients with pain disorders and inflammatory conditions have altered serotonin metabolism.
Flavonoids
Flavonoids are a group of phytochemical compounds found in all vascular plants including fruits and vegetables. They are a part of a larger class of compounds known as polyphenols. Many of the therapeutic or health benefits of colored fruits and vegetables, cocoa, red wine, and green tea are directly related to their flavonoid content. The specially formulated flavonoids found in Theramine cannot be obtained from conventional foods in the necessary proportions to elicit a therapeutic response.
Other Ingredients
Theramine contains the following “inactive” or other ingredients, as fillers, excipients, and colorings: Gelatin, Silicon Dioxide, Tricalcium Phosphate, Vegetable Magnesium stearate, Cellulose, FDandC Blue#1, FDandC Red #3, Titanium Dioxide.
Physical Description
Theramine is a yellow to light brown powder. Theramine contains Gamma Aminobutyric Acid, Choline Bitartrate, Whey Protein Hydrolysate, L-Arginine, L-Histidine HCL, L-Glutamine, Theobromine, Griffonia Seed, Grape Seed, L-Serine, and Cinnamon. Each capsule consists of a proprietary blend of these ingredients in an amount of 366mg or 732mg per two (2) capsule dose. -
CLINICAL PHARMACOLOGY
CCLINICAL PHARMACOLOGY
Mechanism of Action
Theramine acts by restoring and maintaining the balance of the neurotransmitters GABA, nitric oxide, serotonin, and acetylcholine that are associated with pain disorders and inflammatory conditions. Theramine stimulates the production ACTH to reduce inflammation.
Metabolism
The amino acids in Theramine are primarily absorbed by the stomach and small intestines. All cells metabolize the amino acids in Theramine. Circulating tryptophan, arginine and choline blood levels determine the production of serotonin, nitric oxide, and acetylcholine.
Excretion
Theramine is not an inhibitor of cytochrome P450 1A2, 2C9, 2C19, 2D6, or 3A4. These isoenzymes are principally responsible for 95% of all detoxification of drugs, with CYP3A4 being responsible for detoxification of roughly 50% of drugs. Amino acids do not appear to have an effect on drug metabolizing enzymes. - INDICATIONS & USAGE
-
CLINICAL STUDIES
CLINICAL EXPERIENCE
Administration of Theramine has demonstrated significant reduction in symptoms of pain and inflammation in patients with acute and chronic pain when used
for the dietary management of the metabolic processes associated with pain disorders and inflammatory conditions. Administration of Theramine results in
the induction and maintenance of pain relief in patients with pain disorders and inflammatory conditions.
- CONTRAINDICATIONS
-
ADVERSE REACTIONS
ADVERSE REACTIONS
Ingestion of L-Tryptophan, L-Arginine, or Choline at high doses of up to 15 grams daily is generally well tolerated. The most common adverse reactions of higher
doses — from 15 to 30 grams daily — are nausea, abdominal cramps, and diarrhea. Theramine contains less than 1 gram per dose of amino acids however, some patients
may experience these symptoms at lower doses. The total combined amount of amino acids in each Theramine capsule does not exceed 300 mg. - DRUG INTERACTIONS
-
OVERDOSAGE
OVERDOSE
There is a negligible risk of overdose with Theramine as the total amount of amino acids in a one month supply (90 capsules) is less than 30 grams. Overdose symptoms may include diarrhea, weakness, and nausea.
POST-MARKETING SURVEILLANCE
Post-marketing surveillance has shown no serious adverse reactions. Reported cases of mild rash and itching may have been associated with allergies to Theramine flavonoid ingredients, including Cinnamon, Cocoa, and Grape Seed. These reactions were temporary, transient in nature and subsided within 24-hours.
-
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION
Recommended Administration
For the dietary management of the metabolic processes associated with pain disorders and inflammatory conditions. Take two (2) capsules every four hours or as directed by physician. As with most amino acid formulations Theramine should be taken without food to increase the absorption of key ingredients.
-
HOW SUPPLIED
How Supplied
Theramine is supplied in purple and white, size 0 capsules in bottles of 60 and 90 capsules.
Physician Supervision
Theramine is a Medical Food product available by prescription only and may be used per FDA law, and product labeling only while the patient is under ongoing physician supervision.
U.S. patent pending
Manufactured by Arizona Nutritional Supplements, Inc. Chandler AZ 85225
Distributed exclusively by Physician Therapeutics LLC, a wholly owned subsidiary of Targeted Medical Pharma Inc. Los Angeles, CA
www.ptlcentral.com NDC: 68405-008-02 NDC: 68405-008-03
- STORAGE AND HANDLING
-
PRINCIPAL DISPLAY PANEL
68405-008-02 Directions for use: Must be administered under physician supervision. For adults only. As a Medical Food, take one (1) or two (2) capsules every four hours or as directed by physician. For the dietary management of Myalgia. Contains no added sugar, starch, wheat, yeast, preservatives, artificial flavor. Storage: Keep tightly closed in a cool dry place 8-32°C (45-90°F), relative humidity, below 50%. Warning: Keep this product out of the reach of children. NDC# 68405-008-02 LOT# 007007 PHYSICIAN THERAPEUTICS THERAMINE Medical Food Rx only 60 Capsules Ingredients: Each serving (per 2 capsules) contains: Proprietary Amino Acid Formulation Whey Protein Hydrolysate (milk sourced isolate), L-Arginine (as L-Arginine HCl), L-Histidine HCI, L-Glutamine, L-Serine Gamma Amino Butyric Acid Choline Bitartrate Griffonia Seed Extract (5-HTP) Cocoa Extract (6% Theobromine) Grape Seed Extract (85% Polyphenols) Cinnamon (bark) Other Ingredients: Gelatin, Tricalcium Phosphate, Silicon Dioxide, Magnesium Stearate, Microcrystalline Cellulose, Titanium Dioxide, FDandC Red #3, FDandC Blue #1. Distributed exclusively by: Physicians Therapeutics A Division of Targeted Medical Pharma, Inc. Los Angeles, CA 90077 www.ptlcentral.com US Patent 7,582,315; 7,585,523; 7,595,067; 7,601,369. LOT 007007 EXP 08/13
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
THERABENZAPRINE-60
cyclobenzaprine hydrochloride, .gamma.-aminobutyric acid kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68405-580 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68405-580-26 1 in 1 KIT Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 60 Part 2 1 BOTTLE 60 Part 1 of 2 CYCLOBENZAPRINE HYDROCHLORIDE
cyclobenzaprine hydrochloride tabletProduct Information Item Code (Source) NDC: 52959-042(NDC: 0603-3079) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CYCLOBENZAPRINE HYDROCHLORIDE (UNII: 0VE05JYS2P) (CYCLOBENZAPRINE - UNII:69O5WQQ5TI) CYCLOBENZAPRINE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HYPROMELLOSES (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Product Characteristics Color yellow (YELLOW) Score no score Shape ROUND Size 7mm Flavor Imprint Code 2632;V Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52959-042-60 60 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077797 05/15/2011 Part 2 of 2 THERAMINE 60
gaba capsuleProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength .GAMMA.-AMINOBUTYRIC ACID (UNII: 2ACZ6IPC6I) (.GAMMA.-AMINOBUTYRIC ACID - UNII:2ACZ6IPC6I) .GAMMA.-AMINOBUTYRIC ACID 100 mg Inactive Ingredients Ingredient Name Strength GELATIN (UNII: 2G86QN327L) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TRICALCIUM PHOSPHATE (UNII: K4C08XP666) POWDERED CELLULOSE (UNII: SMD1X3XO9M) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 3 (UNII: PN2ZH5LOQY) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color purple (PURPLE, WHITE) Score no score Shape CAPSULE Size 20mm Flavor Imprint Code ; Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 60 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Medical Food 05/15/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/15/2011 Labeler - Physician Therapeutics LLC (931940964) Establishment Name Address ID/FEI Business Operations Vintage Pharmaceuticals 825839835 manufacture Establishment Name Address ID/FEI Business Operations Targeted Medical Pharma Inc. 126962740 manufacture Establishment Name Address ID/FEI Business Operations H.J. Harkins Company, Inc. 147681894 repack
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.