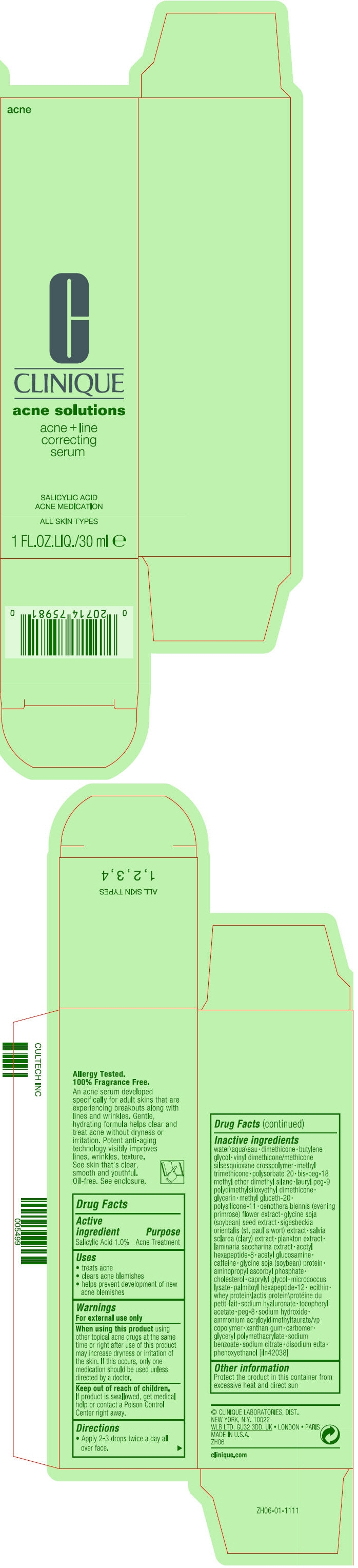
ACNE AND LINE CORRECTING SERUM- salicylic acid lotion
ACNE AND LINE CORRECTING SERUM by
Drug Labeling and Warnings
ACNE AND LINE CORRECTING SERUM by is a Otc medication manufactured, distributed, or labeled by CLINIQUE LABORATORIES LLC, Estee Lauder Companies Inc., PALC, Estee Lauder Cosmetics Ltd., Whitman Laboratories Ltd., Estee Lauder N.V., The Estee Lauder Inc, Northtec LLC, PADC 1. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
-
Inactive ingredients
water\aqua\eau dimethicone butylene glycol vinyl dimethicone/methicone silsesquioxane crosspolymer methyl trimethicone polysorbate 20 bis-peg-18 methyl ether dimethyl silane lauryl peg-9 polydimethylsiloxyethyl dimethicone glycerin methyl gluceth-20 polysilicone-11 oenothera biennis (evening primrose) flower extract glycine soja (soybean) seed extract sigesbeckia orientalis (st. paul's wort) extract salvia sclarea (clary) extract plankton extract laminaria saccharina extract acetyl hexapeptide-8 acetyl glucosamine caffeine gylcine soja (soybean) protein aminopropyl ascorbyl phosphate cholesterol caprylyl glycol micrococcus lysate palmitoyl hexapeptide-12 lecithin whey protein\lactis protein\protéine du petit-lait sodium hyaluronate tocopheryl acetate peg-8 sodium hydroxide ammonium acryloyldimethyltaurate/vp copolymer xanthan gum carbomer glyceryl polymethacrylate sodium benzoate sodium citrate disodium edta phenoxyethanol [iln42038]
- Other information
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 30 ml Bottle Carton
-
INGREDIENTS AND APPEARANCE
ACNE AND LINE CORRECTING SERUM
salicylic acid lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 49527-053 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) dimethicone (UNII: 92RU3N3Y1O) butylene glycol (UNII: 3XUS85K0RA) methyl trimethicone (UNII: S73ZQI0GXM) polysorbate 20 (UNII: 7T1F30V5YH) bis-peg-18 methyl ether dimethyl silane (UNII: OEB4R3WW9C) lauryl peg-9 polydimethylsiloxyethyl dimethicone (UNII: 25G622K2RA) glycerin (UNII: PDC6A3C0OX) methyl gluceth-20 (UNII: J3QD0LD11P) dimethicone/vinyl dimethicone crosspolymer (soft particle) (UNII: 9E4CO0W6C5) oenothera biennis flower (UNII: Y1YXJ1M6Z5) soybean (UNII: L7HT8F1ZOD) saccharina latissima (UNII: 68CMP2MB55) acetyl hexapeptide-8 (UNII: L4EL31FWIL) n-acetylglucosamine (UNII: V956696549) caffeine (UNII: 3G6A5W338E) soy protein (UNII: R44IWB3RN5) aminopropyl ascorbyl phosphate (UNII: 290O2PQ83R) cholesterol (UNII: 97C5T2UQ7J) caprylyl glycol (UNII: 00YIU5438U) palmitoyl hexapeptide-12 (UNII: HO4ZT5S86C) whey (UNII: 8617Z5FMF6) hyaluronate sodium (UNII: YSE9PPT4TH) .alpha.-tocopherol acetate (UNII: 9E8X80D2L0) polyethylene glycol 400 (UNII: B697894SGQ) sodium hydroxide (UNII: 55X04QC32I) ammonium acryloyldimethyltaurate/vp copolymer (UNII: W59H9296ZG) xanthan gum (UNII: TTV12P4NEE) carboxypolymethylene (UNII: 0A5MM307FC) sodium benzoate (UNII: OJ245FE5EU) sodium citrate, unspecified form (UNII: 1Q73Q2JULR) edetate disodium anhydrous (UNII: 8NLQ36F6MM) phenoxyethanol (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49527-053-01 1 in 1 CARTON 08/15/2016 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part333D 08/15/2016 Labeler - CLINIQUE LABORATORIES LLC (044475127) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COMPANY, THE 828534516 RELABEL(49527-053) , REPACK(49527-053) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COSMETICS, LTD 244669714 MANUFACTURE(49527-053) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COSMETICS, LTD. 202952982 MANUFACTURE(49527-053) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER N.V. 370151326 MANUFACTURE(49527-053) Establishment Name Address ID/FEI Business Operations LEN-RON MANUFACTURING DIVISION OF ARAMIS INC 809771152 MANUFACTURE(49527-053) Establishment Name Address ID/FEI Business Operations NORTHTEC INC 943871157 RELABEL(49527-053) , REPACK(49527-053) Establishment Name Address ID/FEI Business Operations PADC 1 949264774 RELABEL(49527-053) , REPACK(49527-053) Establishment Name Address ID/FEI Business Operations WHITMAN LABORATORIES, LTD. 216866277 MANUFACTURE(49527-053) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COSMETICS, LTD 255175580 MANUFACTURE(49527-053)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.