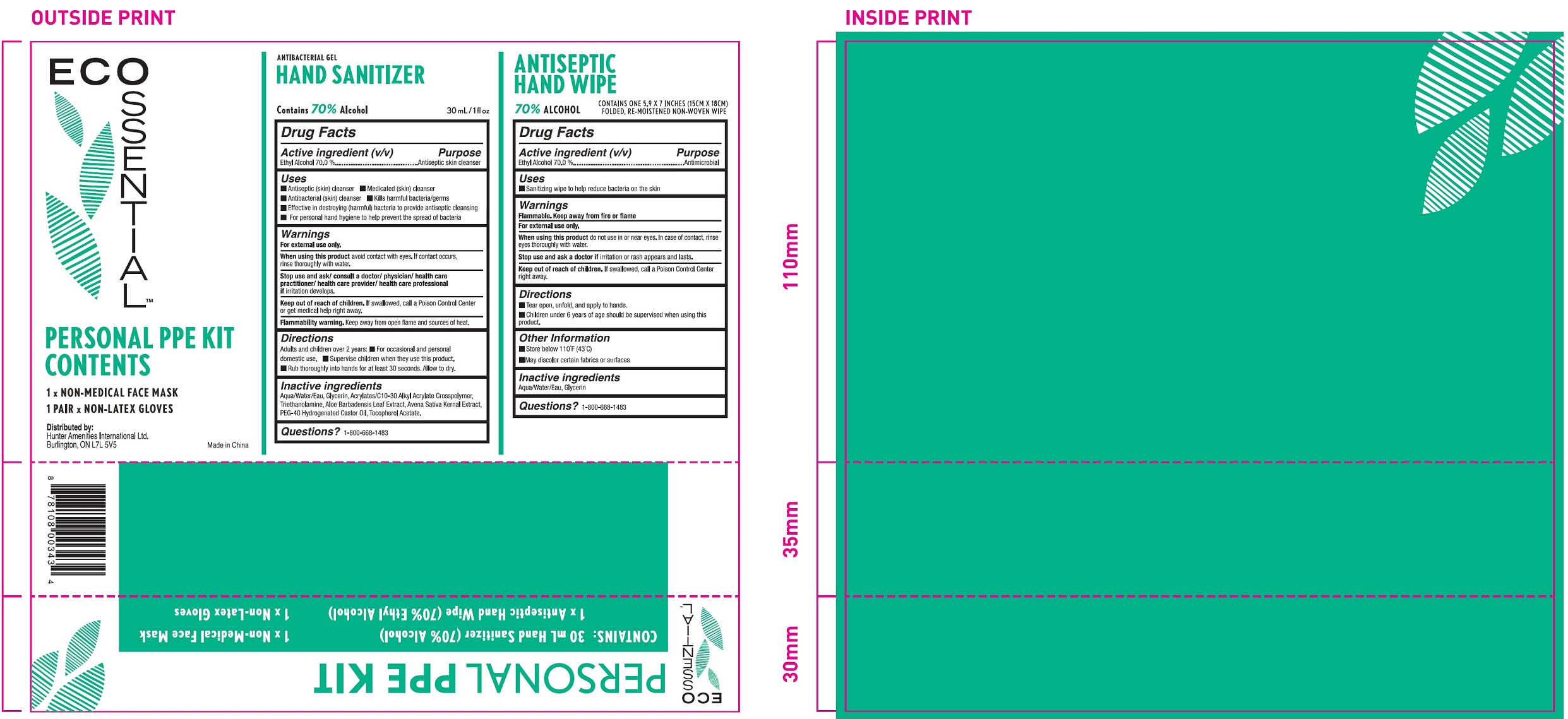
ECOSSENTIAL PERSONAL PPE KIT- alcohol
Hunter Amenities International Limited
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredient (v/v)
Ethyl Alcohol 70.0 %
Purpose
Antiseptic skin cleanser
Uses
- Antiseptic (skin) cleanser
- Medicated (skin) cleanser
- Antibacterial (skin) cleanser
- Kills harmful bacteria/germs
- Effective in destroying (harmful) bacteria to provide antiseptic cleansing
- For personal hand hygiene to help prevent the spread of bacteria
Warnings
For external use only.
When using this product
avoid contact with eyes. If contact occurs, rinse thoroughly with water.
Stop use and ask/ consult a doctor/ physician/ health care practitioner/ health care provider/ health care professional if
irritation develops.
Keep out of reach of children.
If swallowed, call a Poison Control Center or get medical help right away.
Flammability warnings. Keep away from open flame and sources of heat.
Directions
Adults and children over 2 years.
- For occasional and personal domestic use.
- Supervise children when they use this product.
- Rub thoroughly into hands for at least 30 seconds. Allow to dry.
Inactive ingredients
Aqua/Water/Eau, Glycerin, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Triethanolamine,parfum (Fragrance), Aloe Barbadensis Leaf extract,Avena Sativa Kernal Extract, PEG-40 Hydrogenated Castor Oil, Tocopherol Acetate.
Questions?
1-800-668-1483
Active ingredient (v/v)
Ethyl Alcohol 70.0 %
Uses
- Sanitizing wipes to help reduce bacteria on the skin
Warnings
Flammable. Keep away from or flame.
For external use only.
When using this product
do not use in or near eyes. In case of contact, rinse eyes thoroughly with water.
Stop use and ask a doctor if
irritation or rash appears and lasts.
Keep out of reach of children.
If swallowed, call a Poison Control Center right away.
Directions
- Tear open, unfold, and apply to hands
- Children under 6 years of age should be supervised when using this product
Other information
- Store below 110°F (43°C)
- May discolor certain fabrics or surfaces
Inactive Ingredients
Aqua (Water), Glycerin
Questions?
1-800-668-1483
Package Labeling:Kit
Package Labeling:30ml

Package Labeling:Wipe



