CEPACOL EXTRA STRENGTH SORE THROAT TANGERINE- benzocaine and menthol, unspecified form lozenge
Cepacol by
Drug Labeling and Warnings
Cepacol by is a Otc medication manufactured, distributed, or labeled by RB Health (US) LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
-
Warnings
Methemoglobinemia warning
Use of this product may cause methemoglobinemia, a serious condition that must be treated promptly because it reduces the amount of oxygen carried in blood. This can occur even if you have used this product before. Stop use and seek immediate medical attention if you or a child in your care develops:
- pale, gray, or blue colored skin (cyanosis)
- headache
- rapid heart rate
- shortness of breath
- dizziness or lightheadedness
- fatigue or lack of energy
Allergy alert
Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine, or other 'caine' anesthetics.
Sore throat warning
If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, swelling, nausea, or vomiting, consult a doctor promptly.
Stop use and ask a doctor or dentist if
- sore mouth symptoms do not improve in 7 days
- irritation, pain, or redness persists or worsens
- swelling, rash, or fever develops
- Directions
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
-
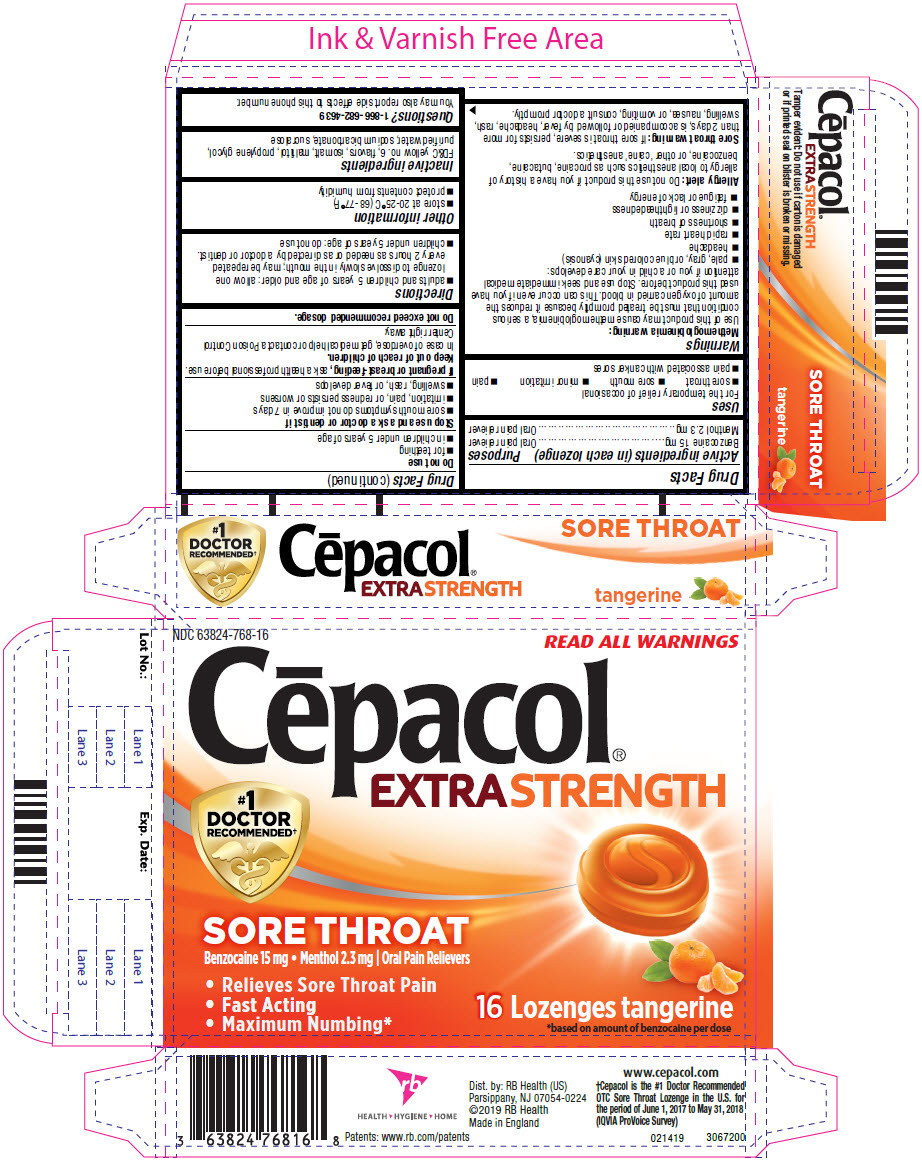
PRINCIPAL DISPLAY PANEL - 16 Lozenge Blister Pack Carton
NDC: 63824-768-16
READ ALL WARNINGS
Cēpacol®
EXTRA STRENGTH#1
DOCTOR
RECOMMENDED†SORE THROAT
Benzocaine 15 mg Menthol 2.3 mg | Oral Pain Relievers
- Relieves Sore Throat Pain
- Fast Acting
- Maximum Numbing*
16 Lozenges tangerine
*based on amount of benzocaine per dose
-
INGREDIENTS AND APPEARANCE
CEPACOL EXTRA STRENGTH SORE THROAT TANGERINE
benzocaine and menthol, unspecified form lozengeProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 63824-768 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Benzocaine (UNII: U3RSY48JW5) (Benzocaine - UNII:U3RSY48JW5) Benzocaine 15 mg Menthol, Unspecified Form (UNII: L7T10EIP3A) (Menthol, Unspecified Form - UNII:L7T10EIP3A) Menthol, Unspecified Form 2.3 mg Inactive Ingredients Ingredient Name Strength FD&C yellow no. 6 (UNII: H77VEI93A8) isomalt (UNII: S870P55O2W) maltitol (UNII: D65DG142WK) propylene glycol (UNII: 6DC9Q167V3) water (UNII: 059QF0KO0R) sodium bicarbonate (UNII: 8MDF5V39QO) sucralose (UNII: 96K6UQ3ZD4) Product Characteristics Color ORANGE Score no score Shape ROUND Size 18mm Flavor TANGERINE Imprint Code S Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63824-768-16 2 in 1 CARTON 07/15/2015 1 8 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part356 07/15/2015 Labeler - RB Health (US) LLC (081049410)
Trademark Results [Cepacol]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CEPACOL 98112933 not registered Live/Pending |
RB Health (US) LLC 2023-08-02 |
 CEPACOL 88792597 not registered Live/Pending |
RB Health (US) LLC 2020-02-11 |
 CEPACOL 78632524 not registered Dead/Abandoned |
J.B. Williams Company, Inc. 2005-05-18 |
 CEPACOL 76689146 3510479 Live/Registered |
RB HEALTH (US) LLC 2008-04-30 |
 CEPACOL 75046415 2410917 Dead/Cancelled |
CEP Holdings, Inc. 1996-01-22 |
 CEPACOL 74642965 2086261 Live/Registered |
RECKITT BENCKISER LLC 1995-03-06 |
 CEPACOL 72029539 0662139 Dead/Expired |
WM. S. MERRELL COMPANY, THE 1957-05-07 |
 CEPACOL 71432309 0383155 Live/Registered |
Wm. S. Merrell Company, The 1940-05-24 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.