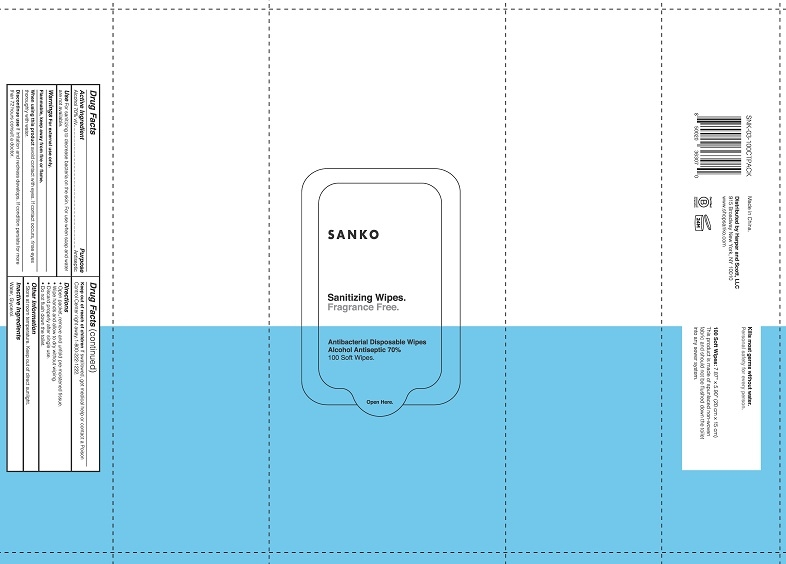
Sanko Sanitizing Wipes by Harper + Scott LLC Sanko Sanitizing Wipes
Sanko Sanitizing Wipes by
Drug Labeling and Warnings
Sanko Sanitizing Wipes by is a Otc medication manufactured, distributed, or labeled by Harper + Scott LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SANKO SANITIZING WIPES- alcohol cloth
Harper + Scott LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Sanko Sanitizing Wipes
Warnings
For external use only.
Flammable, keep away from fire or flame.
When using this product avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
Directions
- Open packet, remove and unfold pre-moistened tissue.
- Wipe hands and allow to dry without wiping.
- Discard properly after single use.
- Do not flush down toilet.
Made in China.
Distributed by Harper and Scott, LLC
915 Broadway New York, NY 10010
www.shopsanko.com
Kills most germs without water. Personal safety for every person.
100 Soft Wipes: 7.87" x 5.90" (20 cm x 15 cm)
This product is made of spunlaced non-woven fabric and should not be flushed down the toilet into any sewer system.
| SANKO SANITIZING WIPES
alcohol cloth |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Harper + Scott LLC (077459979) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.